A multi-center retrospective study of single-port versus multi-port video-assisted thoracoscopic lobectomy and anatomic segmentectomy
Introduction
Video-assisted thoracoscopic surgery (VATS) is now the preferred approach for management of early stage lung cancer (1). Compared with the traditional open surgery, the benefits of VATS include reduced surgical trauma and postoperative pain, shorter hospital stay, less scarring, reduced complication and mortality rates, and similar long-term survival rate (2-5). In the quest for making VATS more patient friendly in terms of fewer complications and better cosmesis, VATS has evolved from using 3–4 ports toward a single-port (SP) approach (6). Similar to when multi-port (MP) VATS was introduced, there is skepticism regarding the feasibility of this SP approach (7). For any new technique to be adopted, it has to offer comparable safety and feasibility to already established techniques, as well as the potential to confer further benefits. To evaluate the feasibility, safety, and benefits of SP VATS, we conducted a multi-center retrospective study to compare the outcomes of patients who underwent SP and MP VATS lobectomy and anatomical segmentectomy.
Methods
Clinical data
Retrospective data of patients who received VATS anatomic lobectomy or segmentectomy at Shanghai Chest Hospital, Korea University Guro Hospital, Affiliated Hospital of National Taiwan University, University of Hong Kong Queen Mary Hospital and Shenzhen Hospital were collected. The patients were divided into a SP group and MP group according to the surgical approach. The decision to perform SP VATS or MP VATS for each patient was determined at the surgeon’s discretion before operation.
Clinical data such as age, gender, clinical cancer stage, respiratory comorbidities (chronic obstructive lung disease, asthma, pneumonia, emphysema, etc.), cardiovascular comorbidities (hypertension, atrial fibrillation, coronary artery disease, etc.), diabetes mellitus, preoperative spirometry [forced expiratory volume in 1 second (FEV1) as a percentage of predicted, carbon monoxide diffusing capacity (DLCO) as a percentage of predicted], history of other malignancy, and previous chest operations were collected and compared between the groups. To compare the perioperative surgical outcomes, intraoperative data (operation time, intraoperative blood loss, thoracoscopic findings such as pleural adhesions, conversion to MP or thoracotomy), postoperative data (including postoperative extubation time, hospitalization time, perioperative complications, and mortality) were reviewed and analyzed. Patients with surgically resectable lung cancer who were intentionally treated with thoracoscopic lobectomy or segmentectomy were included in the study. Patients were classified into SP group and MP group according the surgical approach initially attempted to. Those who had a conversion intraoperatively were also included based on the intention-to-treat principle but not for the pain score analysis. Patients who underwent wedge resections, pneumonectomy, bilobectomy, sleeve resection, or chest wall resection were excluded. Those who received induction chemotherapy or chemoradiotherapy before surgery were also excluded from the study.
Pain scoring and postoperative pain management
Postoperative pain was evaluated for the first 3 postoperative days utilizing a visual analog scale (VAS) (8,9). Scores ranged between 0 and 10, where 0 was no pain and 10 was the worst pain imaginable. After surgery, the patients were routinely provided with a patient-controlled intravenous analgesia for the first day after operation. On the second day, the patient-controlled intravenous analgesia was removed. The patients were given non-steroidal anti-inflammatory drugs like tylox or celebrex if the VAS score was more than 4 or the patients asked for analgesic agents. If the patients got no relief of pain, opioid analgesics like tramadol hydrochloride or fentanyl were provided.
Surgical technique
Double-lumen endotracheal intubation, and single lung ventilation under general anesthesia was used in all cases. As this study was retrospective and multicenter, the surgical operations varied slightly with respect to the positions of the ports. The bi-portal approach used a 1.5 cm incision at the seventh intercostal space at the level of the anterior axillary line for the camera, and a 3–5 cm incision at the fourth or third intercostal space as the operating port. The 3-port method used an additional 2 cm port at the level of the posterior axillary line at the seventh intercostal space for the assistant. For the 4-port approach, a 1–2 cm incision was made between the camera port and the assistant port. The SP approach required only a 4 to 6 cm incision at the fifth intercostal space at the anterior axillary line. But in a few number of cases, the incision was made in the fourth intercostal spaces, especially the upper lobe lobectomy. A wound protector was routinely applied for the operation port in MP VATS and the single incision in SP VATS. Energy devices, suction devices, vascular clips, hook-type monopolar electrocautery and a 30-degree angled thoracoscope were routinely used in the both groups. A trocar was inserted in the camera port in MP VATS while it was not used in SP VATS. Pulmonary veins, pulmonary arteries, bronchial, interlobar fissures, and intersegmental fissures were dissected with disposable mechanical staplers or hemlocks. The resected specimen was retrieved in a specimen bag. In cases whereas intraoperative frozen section showed the lesion to be malignant, systematic lymph node dissection or sampling was performed.
If an additional port was needed during operation, a 1–2 cm incision was added where the surgeon considered appropriate. Conversion to mini or full thoracotomy was usually performed by extending the single incision in the SP VATS and operation port in MP VATS.
After the completion, the chest tube was removed if there was no air leakage with the daily chest tube drainage volume smaller than 300 mL and satisfactory lung expansion on chest X-rays. And the patient was discharged with no fever and sufficient lung expansion on the chest X-ray the day after the removal of the chest tube.
Statistical analysis
Statistical analysis was performed using the SPSS19.0 software. For quantitative variables, the t-test was used for evaluation if data were normally distributed. Non-normally distributed data were analyzed with the Mann-Whitney test. For qualitative variables, we examined with the chi square test or Fisher’s exact test when appropriate. A value of P<0.05 was considered statistically significant.
Results
Data of 458 patients who underwent lobectomy or anatomical segmentectomy between 2013 and 2014 were included in the analysis. Of the patients, 166 (36.2%) had a SP procedure and 292 (63.8%) had a MP procedure. The patients were comprised of 231 males and 277 females with an average age of 62.5 years. Of the procedures, 120 were performed at Shanghai Chest Hospital (including 11 SP and 109 MP), 155 at National Taiwan University Hospital (including 57 SP and 98 MP), 106 St Mary’s Hospital affiliated to the Hong Kong University and Shenzhen Hospital (including 24 SP and 82 MP), and 77 at the Korea University Guro Hospital (including 74 SP and 3 MP). Altogether, lobectomy was performed in 303 patients and anatomical segmentectomy in 155 cases. The demographics characteristics of the patients between SP group and MP group are list in Table 1.
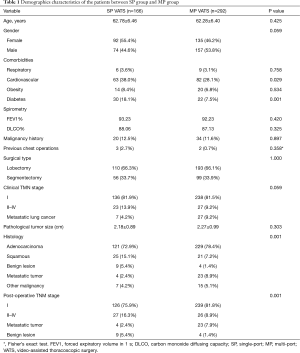
Full table
No significant differences were noted in age (P=0.425), gender (P=0.059), respiratory system comorbidities (P=0.758), obesity (P=0.534), history of malignancy (P=0.897), and previous chest operations (P=0.358) between the two groups. There were a higher number of patients with diabetes (P=0.001) and cardiovascular disease (P=0.029) in the SP group than in the MP group.
The proportion of patients in the MP group who were diagnosed with metastatic lung cancer was slightly higher than in the SP group (MP vs. SP, 9.2% vs. 4.2%; P=0.048). The percentage of clinical stage I lung cancer patients was almost the same between the two groups (MP vs. SP, 81.5% vs. 81.9%; P=0.911). With respect to the histology and pathological TNM stage, more patients in the SP group had squamous cell carcinoma (SP vs. MP, 15.1% vs.7.2%; P=0.007) and benign lesions (SP vs. MP, 5.4% vs.1.4%; P=0.012) than in the MP group, while more patients in the MP group had metastatic carcinoma (SP vs. MP, 2.4% vs.8.9%; P=0.017). Among patients with pathologically proven primary lung cancer, there were significantly higher stage tumors in the SP group than in the MP group (SP vs. MP, 17.6% vs. 9.8%, P=0.02).
The surgical characteristics and lymph node harvested between SP and MP group are shown in Table 2. There were no statistically significant differences in the number of stations (P=0.278) and the number of lymph nodes (P=0.564) collected between the two groups. Both groups had a favorable R0 resection rate. The operation time of SP VATS was slightly longer than that of MP VATS (P=0.042). In the SP group, more patients had over 500 mL blood loss than in the MP group (13.2% vs. 3.1%; P <0.001), while the conversion rate in the MP group was even higher than in the SP group (P=0.018). In the SP group, two patients needed an additional port, and one was converted to full thoracotomy. In the MP group, fourteen patients were converted to mini thoracotomy, two patients needed an additional port, and four were converted to full thoracotomy.
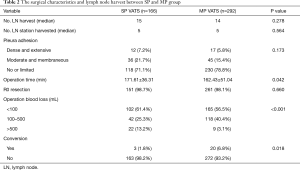
Full table
With regard to the postoperative recovery (Table 3), chest tube removal time (P=0.012) and postoperative hospitalization time (P=0.005) were shorter after SP VATS than after MP VATS. There were 69 postoperative complications in total: 17 complications in the SP group (16.4%) and 47 complications in the MP group (13.0%). The most common complications were air leak, pneumonia, arrhythmia, and recurrent nerve palsy. There were 7 postoperative mortalities. Five were due to postoperative pneumonia and acute lung injury. One patient had interstitial lung disease before surgery, and died of respiratory failure. The last patient died of acute pulmonary embolism. The incidence of perioperative complications (P=0.246) and mortality rate (P=0.414) were not different between the two groups.
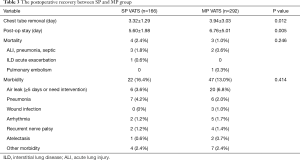
Full table
Pain scores of the SP group were significantly lower than that of the MP group on the first (P=0.014) and second (P=0.006) but not on the third postoperative day (Table 4).
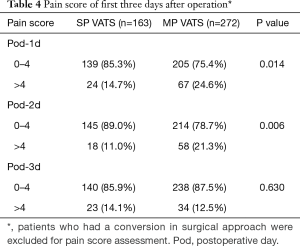
Full table
Discussion
SP VATS is among the most recent innovations in the field of minimally invasive thoracic surgery. The history of SP VATS dates back to 2004, when Rocco described a SP thoracoscopic pulmonary wedge resection (10). The technique was further refined by Gonzalez-Rivas, and indications for other chest disease gradually developed in recent years (6,11-14).
There are many published studies regarding the use of SP VATS (15-18) in the diagnosis and treatment of pulmonary diseases. But these studies are mostly single center research, or small case series. In view of the increasing use of SP VATS, we conducted a multicenter retrospective study. The five hospitals that participated in the study were large referral medical centers, representing institutions of advanced surgical technology for diagnosis and treatment.
Operation time, intraoperative bleeding, and the conversion rate to open surgery are important parameters reflecting the safety of a surgical procedure. The current data showed that the operation time of the SP group was longer than that of the MP group. The technical complexity of SP approach is greater than that of MP VATS, requiring a learning phase. This technique involves the use of a single access point that allows the introduction of 3 to 4 instruments through one incision. During surgery, triangulation of the instruments requires vision to be central, and to have one working instrument on either side. Triangulation ensures the most comfortable working position for the surgeon ergonomically, and is always present in conventional MP VATS approaches. However, it is difficult to achieve triangulation in SP VATS. Moreover, the amount of bleeding above 500 mL in the SP VATS group was higher than that of the MP VATS group. It may be due to the learning curve at this time point. This suggests that SP VATS is technically more demanding than MP VATS. With increased experience, we became more familiar with this approach and more confident in handling the intraoperative accidents as the number of patients undergoing SP VATS increased. Unlike the torsional angle in the conventional MP approach, SP VATS provides a new anatomical and direct view which is similar to open thoracotomy, which may help the surgeons to shorten their learning curve for SP VATS. Fortunately, in the current study, no fatal hemorrhage occurred in any patient. And there was no postoperative bleeding or need for re-exploration for bleeding. In addition, the conversion rate was not higher in the SP group than MP group. Therefore, we consider that although SP VATS may require greater surgical skill than MP VATS, it is safe and feasible in experienced hands.
Systematic lymph node dissection is important for accurate staging and radical resection of lung cancer, and is also a useful index for evaluation of a surgical technique. To date, results comparing number and station of lymph nodes between SP VATS and MP VATS have been discordant (15,17,19). Our data showed that the number of lymph nodes harvested by SP VATS was not different from MP VATS. This suggests that effective lymph node dissection could be achieved through SP VATS, which is consistent with a prior report by Liu et al. (19).
Regarding the surgical outcomes, there was no difference in postoperative complication or mortality rates between the two groups. SP VATS provided superior results with respect to chest tube removal time and the length of hospital stay. SP VATS was also associated with lower pain scores after surgery in our study. Postoperative pain management is strongly associated with pulmonary complications (20). Hirai et al. (17) retrospectively analyzed 20 cases of MP VATS and 60 cases of SP VATS and reported that postoperative pain was lower in the SP group on postoperative days 3, 7, and 13, with less need for analgesics. We hypothesize that in SP VATS, camera and surgical devices are inserted nearly perpendicularly to the plane of the chest incision, causing less compression on the ribs. And only one intercostal nerve is affected. In contrast, in MP VATS with more incisions at different intercostals spaces, there are more extensive damage to intercostal nerves at several sites and therefore severer paresthesia. This is especially the case in patients with a barrel-shaped thorax, low height and severe obesity, and the intercostal nerve may be readily damaged, as reported by Hirai et al. (17). Although in our study there was not statistically difference between the two groups regarding the incidence of postoperative complications, duration of chest drainage and length of hospital stay in the MP group were longer than in the SP group. This may be associated with the impairment of effective breathing and coughing due to more chest pain after surgery (20). We hypothesize that less chest pain might help the patients to an earlier ambulation and shorter recovery time.
Our study was carried out at multiple centers with expertise in minimally invasive surgery. However, there are intrinsic biases associated with its multi-center and retrospective nature. Patients were not randomized for procedure allocation as true randomization is extremely difficult in the clinical setting. And surgical skills and position of incisions varies from different institution. Patient follow-up was short and VAS pain scores were obtained only in the first 3 postoperative days. The cost effectiveness of SP VATS was not studied. As equipment become more sophisticated, they maybe more expensive as compared to standard thoracoscopic instruments. A new and innovative surgical technique normally involves added expenditures. With the development of a new technique, the accompanying learning curve may expose patients to risk. The safety of patients undergoing a new surgical technique should be paramount. Whether the increased cost translates into better outcomes for the patient in terms of faster and better recovery is yet to be determined.
Today, SP VATS stands where minimally invasive surgery stood about two decades ago. It faces almost the same challenges and skepticism faced by the conventional thoracoscopic surgery. With ongoing changes and advances in the field of minimally invasive surgery, long-term follow-up and controlled trials are required to determine if SP VATS could be a meaningful and lasting technique.
In conclusion, this multi-center study shows that SP VATS is a safe and feasible approach, with comparable preoperative outcomes but less early postoperative pain to MP VATS. Larger, prospective trials and long-term follow-up results are still needed to further validate the surgical feasibility and oncological effectiveness of this approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board of Shanghai Chest Hospital approved this study, and patients’ informed consent was waived because of the retrospective nature of the study design.
References
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Higuchi M, Yaginuma H, Yonechi A, et al. Long-term outcomes after video-assisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer. J Cardiothorac Surg 2014;9:88. [Crossref] [PubMed]
- Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg 2008;85:S719-28. [Crossref] [PubMed]
- Puri V, Meyers BF. Video-assisted thoracoscopic surgery lobectomy for lung cancer. Surg Oncol Clin N Am 2013;22:27-38. v. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Sihoe AD. Reasons not to perform uniportal VATS lobectomy. J Thorac Dis 2016;8:S333-43. [PubMed]
- Jensen MP, Karoly P, O'Riordan EF, et al. The subjective experience of acute pain. An assessment of the utility of 10 indices. Clin J Pain 1989;5:153-9. [Crossref] [PubMed]
- Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S240-52. [Crossref] [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Mendez L, et al. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg 2012;42:e169-71. [Crossref] [PubMed]
- Gonzalez-Rivas D, de la Torre M, Fernandez R, et al. Video: Single-incision video-assisted thoracoscopic right pneumonectomy. Surg Endosc 2012;26:2078-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:E2. [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [Crossref] [PubMed]
- Tamura M, Shimizu Y, Hashizume Y. Pain following thoracoscopic surgery: retrospective analysis between single-incision and three-port video-assisted thoracoscopic surgery. J Cardiothorac Surg 2013;8:153. [Crossref] [PubMed]
- Hirai K, Takeuchi S, Usuda J. Single-incision thoracoscopic surgery and conventional video-assisted thoracoscopic surgery: a retrospective comparative study of perioperative clinical outcomes. Eur J Cardiothorac Surg 2016;49 Suppl 1:i37-41. [PubMed]
- Mu JW, Gao SG, Xue Q, et al. A Matched Comparison Study of Uniportal Versus Triportal Thoracoscopic Lobectomy and Sublobectomy for Early-stage Nonsmall Cell Lung Cancer. Chin Med J (Engl) 2015;128:2731-5. [Crossref] [PubMed]
- Liu CC, Shih CS, Pennarun N, et al. Transition from a multiport technique to a single-port technique for lung cancer surgery: is lymph node dissection inferior using the single-port technique? Eur J Cardiothorac Surg 2016;49 Suppl 1:i64-72. [PubMed]
- Denu ZA, Yasin MO, Melekie TB, et al. Postoperative Pulmonary Complications and Associated Factors among Surgical Patients. J Anesth Clin Res 2015;6:554.


