Prognostic role of a comprehensive geriatric assessment on the management of elderly patients with advanced non-small cell lung cancer (NSCLC): a pooled analysis of two prospective phase II trials by the GFPC Group
Introduction
Lung cancer is the leading cause of cancer-related death worldwide (1). Eighty-five percent of diagnosed lung cancer patients have non-small cell lung cancer (NSCLC) (1) and 70% of them are diagnosed at an advanced stage when systemic therapy is the standard option. Median age at diagnosis is 70 years (2) and lung cancer is therefore a disease of older adults (3). Age is an important factor in NSCLC management decisions, because of the complex interplay between normal age-related decline and co-morbidities. Elderly patients are under-represented in clinical trials, making it difficult to predict the tolerability and outcome of chemotherapy (4). In the routine clinical setting, physicians generally use a combination of performance status (PS) and organ function to select patients for chemotherapy. PS is a strong predictor of outcomes in older cancer patients, especially those with advanced-stage NSCLC (5). However, successful treatment of elderly patients also depends on their physical, cognitive and nutritional status, mobility, and social support. For this reason, more formal geriatric assessments have been developed (6-12). Although there is no consensus method for identifying frail or pre-frail older patients, a number of clinical screening tests are now available (13). Comprehensive geriatric assessment (CGA) consists of a set of tools for assessing cognitive function, psychological, functional and nutritional status, co-morbidities, and medication. Comorbidities are generally assessed with the Charlson comorbidity index and the Cumulative Illness Rating Scale for Geriatrics (CIRS-G). Several studies have examined the impact of comorbidities on overall survival (OS), in-hospital mortality, hospitalizations, adverse effects, quality of life, and treatment allocation (14).
In elderly patients with advanced-stage NSCLC, several phase II trials and a large phase III trial failed to show a clear benefit of using the CGA for allocation treatment (5,15-19). In addition, the CGA has been criticised for being time-consuming, cumbersome and not standardised, and its real influence on treatment decisions in clinical practice has been questioned (12).
The aim of this study was to estimate the prognostic role of the different GCA domains on OS in elderly patients with advanced-stage NSCLC, by analyzing individual data from two phase II trials.
Methods
Data
We pooled individual data from two prospective, multicenter, open-label randomized phase II trials in chemotherapy-naive patients over 65 years old with advanced-stage NSCLC. GFPC 0504 involved fit patients (15) and GFPC 0505 frail patients (16), as identified with a CGA. Both trials compared first-line chemotherapy (gemcitabine plus docetaxel for fit patients, gemcitabine alone for frail patients) followed by second-line erlotinib versus the reverse strategy (erlotinib followed by chemotherapy). In both studies the primary endpoint was the time to second-line progression (PFS2). OS was a secondary endpoint.
CGA
All the patients were assessed at inclusion with a CGA by their regular clinicians. The protocol included no specific interventions to improve health disorders identified by the CGA.
The CGA (Table 1) included situational, outdoor, social environments, home ergonomics, cognitive and sensory functions (Folstein MMSE), emotional balance, self-confidence and mood, including a depression scale (GDS 5), a nutritional assessment (mini MNA), a quality-of-life scale (IRIS), pain assessment, activities of daily living (ADL) and instrumental activities of daily living (IADL), sphincter control, and motor skills (including falls in the past year and a get-up-and-go test).

Full table
Patients were classified as fit or frail according age, the number of comorbidities, PS (Table 2).
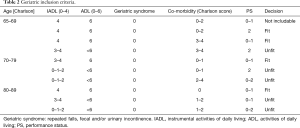
Full table
Statistical analysis
Standard descriptive statistics were used. Quantitative data were expressed as the population, number, mean, standard deviation and range; qualitative data were expressed as the population, number and frequency. All tests were two-sided, and significance was assumed at P<0.05. Quantitative variables were compared with Student’s t-test or with Wilcoxon’s test when the groups were too small or the data were not normally distributed. Qualitative parameters were compared with the Chi2 test for theoretical group sizes above 5, and otherwise with Fisher’s test.
The endpoint of this prognostic study was OS, defined as the time between randomisation and death from any cause. Patients who did not die were censored at the date of last follow-up. Survival rates and their 95% confidence intervals were estimated with the Kaplan-Meier method. The log-rank test was used to compare survival curves. Parameters significantly associated with OS in univariate analysis (P<0.05) were further tested in multivariate analysis. Hazard ratios (95% confidence interval) were estimated with Cox’s proportional hazards regression model (multivariate analysis), using the lowest-risk group as reference. All tests were two-sided. SAS software version 8.2 was used (Institute INC, Carry, USA).
The protocol was approved by an independent ethics committee in Marseille (N 05/31 and 05/32, 14/02/2015) on behalf of all participating centers, and the study complied with Good Clinical Practices and the Helsinki Declaration. According to French regulations, all the participants gave informed consent before taking part to the study.
Results
From May 2006 to January 2010, respectively 100 and 94 patients were eligible for trials 0504 and 0505. These 194 patients constituted the sample for the present pooled analysis. Mean age was 77 years (66–88 years), and 135 (70%) of the patients were men. PS was 0 in 71 cases (36%) (Table 3). At CGA assessment, respectively 166 (85.6%) and 176 (90.7%) patients had ADL and IADL scores of 3 and 4, 129 (66%) had a Charlson score of 0 or 1, 175 (90.2%) had a mini mental status (MMS) score of >30, 146 (75%) had a motor score of 0 or 1, and 161 (82.9%) had a normal nutritional score (Table 4)
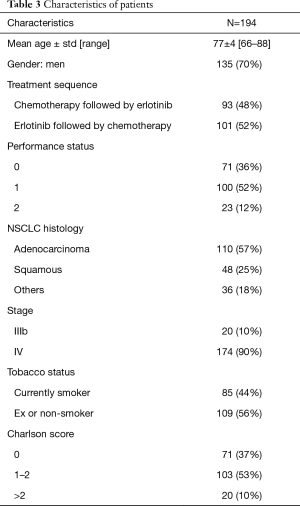
Full table
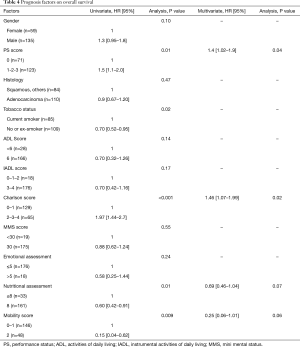
Full table
The most frequent co-morbidities, based on the Charlson score, were COPD (29.5%) and peripheral arterial disease (18.1%). Ninety-three patients (48%) received chemotherapy followed by erlotinib, while the remaining 101 (52%) patients received erlotinib followed by chemotherapy. No significant difference between the arms was observed in either trial in terms of PFS2 or OS. In the pooled analysis, PFS2 was respectively 6.1 and 4.9 months in patients who received chemotherapy first and erlotinib first, and the respective OS was 7.1 and 5.9 months. Median OS was 6.3 months in the pooled population. Prognostic factors of OS in univariate analysis were PS (P=0.01), smoking status (P=0.02), the Charlson score (P<0.001), the simplified Charlson score (P=0.03), the nutritional score (P=0.01), and the level mobility score (P=0.009) (Table 4). In multivariate analysis, PS (P=0.04) and the Charlson score (P=0.02) were independently prognostic of OS, while the nutritional score [HR: 0.69 (0.46–1.04), P=0.07] and the mobility score [HR: 0.25 (0.06–1.01), P=0.06] were close to significance.
Discussion
In this pooled analysis of two trials involving elderly patients with advanced-stage NSCLC, in which the choice of chemotherapy was guided by a CGA, PS and the Charlson co-morbidity score were prognostic of OS, while nutritional and mobility scores were close to significance. These results confirm that PS is a major prognostic factor for OS in lung cancer, as in other malignancies. The role of PS in this setting was recently confirmed in a large French prospective cohort study (20). As in other advanced cancers (21,22), the number of co-morbidities is also significantly associated with OS in advanced-stage NSCLC. The link between co-morbidities and OS is more readily found in homogeneous elderly NSCLC populations (23-25). A joint analysis of two prospective randomized trials of systemic chemotherapy (adjuvant/palliative setting) in a total of 1,255 patients with NSCLC showed a clear association between the Charlson comorbidity index and OS (14). The high prevalence of smoking among patients with advanced NSCLC may help to explain the impact of co-morbidities, especially cardiovascular disease (13).
This analysis included fit and no-fit patients but there is no consensus on the tools used for the different CGA domains. The cut-off of each tool remains also controversial. Here (15,16) the cut-offs were choose to select elderly patients with advanced NSCLC able to receive a mono or a doublet of chemotherapy. This explain why for MMS for example the cut off was 30, instead of a cut off of 24 used in another’s studies to assess the cognitive function of elderly patients without cancer.
We found no impact of several domain of CGA on OS among patients with advanced NSCLC. One possible explication is the exclusion of patients with poor functional status, i.e., patients with a geriatric syndrome, PS 3 or 4, poor MMS. Most of the rare studies in this area did not base treatment choices on geriatric criteria but included a geriatric assessment before chemotherapy. In a prospective multicenter study, previously untreated patients over 70 years of age scheduled for first-line chemotherapy for various malignancies were assessed with an abbreviated CGA, including the Mini-Mental State Exam, Timed Get Up and Go (GUG), ADL, IADL, Mini Nutritional Assessment (MNA), Geriatric Depression Scale (GDS15), and a co-morbidities index. A low MNA score and a long GUG (poor mobility) were associated with a higher risk of early death (<6 months), contrary to ADL and IADL (19). A history of falls was associated with a three-fold higher risk of early death. Our results are in keeping with these data, with a non significant tendency for mobility and nutritional status to be associated with OS. We found only one small, open phase II study of 59 untreated patients over 70 years of age with advanced NSCLC showing that ADL and IADL were significantly related to OS in multivariate analysis (24). No biological or clinical markers of nutritional status in oncology or CGA has been identified (26,27). Depression has been independently linked to poorer treatment adherence and poorer outcomes in cancer patients (5). In a retrospective analysis of CGA data for 249 consecutive cancer patients aged 70 years or more, an abnormal score on a geriatric depression scale was an independent predictor of poorer OS, but we found not such relationship (5). More recently (17) in a large multicenter, phase III trial, in elderly patients with advanced NSCLC, management allocation on the basis of CGA failed to improve OS but significantly reduced treatment toxicities.
There is currently no agreed alternative to the CGA for patient selection. It would be prudent to prioritise those tools with the best sensitivity (albeit less specific) and those with which physicians are familiar. With good sensitivity and independent prognostic value for 1-year survival, the G8 questionnaire (20) is currently one of the best screening tools for identifying older cancer patients requiring geriatric assessment, and we believe it should be broadly implemented in daily practice. Research must continue to refine the selection of older cancer patients for potentially life-threatening therapy (20,28).
Our analysis has several strengths. In particular, the geriatric assessment was done prospectively before treatment initiation, by the clinician in charge of the patient, in a real-life clinical situation. In addition, the population was restricted to patients with advanced-stage NSCLC, and only two chemotherapy regimens were used.
One limitation of the study was the selection of the patients able to be randomized on analyzed studies, with for example, very few patients with major cognitive problems, or major disabilities. A second limitation was the administration of the CGA by the clinician in charge of the patient and not by a geriatric specialist but our group and the clinicians involved in these studies have an important background on this topic (15-17,29,30).
In conclusion, PS and comorbidities were the main predictors of OS among elderly patients with advanced-stage NSCLC assessed by a CGA and treated by chemotherapy. Large prospective cohorts studies are needed to identify the best tools for guiding the management of elderly patients with advanced-stage NSCLC.
Acknowledgements
This trial was an academic trial conducted by Groupe Français de Pneumo Cancerologie (GFPC). This study received public funding.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol was approved by an independent ethics committee in Marseille (N 05/31 and 05/32, 14/02/2015) on behalf of all participating centers, and the study complied with Good Clinical Practices and the Helsinki Declaration and written informed consent obtained from all patients.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570-7. [Crossref] [PubMed]
- Zauderer MG, Sima CS, Korc-Grodzicki B, et al. Toxicity of initial chemotherapy in older patients with lung cancers. J Geriatr Oncol 2013;4:64-70. [Crossref] [PubMed]
- Hutchins LF, Unger JM, Crowley JJ, et al. Under representation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061-7. [Crossref] [PubMed]
- Kanesvaran R, Li H, Koo KN, et al. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. J Clin Oncol 2011;29:3620-7. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies development and validation. J Chron Dis 1987;40:373-83. [Crossref] [PubMed]
- Gironés R, Torregrosa D, Maestu I, et al. Comprehensive geriatric assessment (CGA) of elderly lung cancer patients: a single-center experience. J Geriatr Oncol 2012;3:98-103. [Crossref]
- Hamaker ME, Jonker JM, de Rooij SE, et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients: a systematic review. Lancet Oncol 2012;13:e437-44. [Crossref] [PubMed]
- Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Coopera tive Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol 2002;20:494-502. [Crossref] [PubMed]
- Wedding U, Ködding D, Pientka L, et al. Physicians’ judgement and comprehensive geriatric assessment (CGA) select different patients as fit for chemotherapy. Crit Rev Oncol Hematol 2007;64:1-9. [Crossref] [PubMed]
- Hamaker ME, Schiphorst AH, Ten Bokkel Huinink D, et al. The effect of a geriatric evaluation on treatment decisions for older cancer patients – a systematic review. Acta Oncol 2014;53:289-96. [Crossref] [PubMed]
- Schulkes KJ, Hamaker ME, van den Bos F, et al. Relevance of a geriatric assessment for elderly patients with lung cancer-a systematic review. Clin Lung Cancer 2016;17:341-349.e3. [Crossref] [PubMed]
- Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screeningand assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol 2013;24:1306-12. [Crossref] [PubMed]
- Asmis TR, Ding K, Seymour L, et al. National Cancer Institute of Canada Clinical Trials Group. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol 2008;26:54-9. [Crossref] [PubMed]
- LeCaer H, Barlesi F, Corre R, et al. A multicentre phase II randomised trial of weekly docetaxel/gemcitabine followed by erlotinib on progression, vs the reverse sequence, in elderly patients with advanced non small-cell lung cancer selected with a comprehensive geriatric assessment (the GFPC 0504 study). Br J Cancer 2011;105:1123-30. [Crossref] [PubMed]
- LeCaer H, Greillier L, Corre R, et al. A multicenter phase II randomized trial of gemcitabine followed by erlotinib at progression, versus the reverse sequence, in vulnerable elderly patients with advanced non-small-cell lung cancer selected with a comprehensive geriatric assessment (the GFPC 0505 study). Lung Cancer 2012;77:97-103. [Crossref] [PubMed]
- Corre R, Greillier L, Le Caër H, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08-02 Study. J Clin Oncol 2016;34:1476-83. [Crossref] [PubMed]
- Maas HA, Janssen-Heijnen ML, Olde Rikkert MG, et al. Comprehensive geriatric assessment and its clinical impact in oncology. Eur J Cancer 2007;43:2161-9. [Crossref] [PubMed]
- Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of Early Death Risk in Older Patients Treated With First-Line Chemotherapy for Cancer. J Clin Oncol 2012;30:1829-34. [Crossref] [PubMed]
- Soubeyran P, Bellera C, Goyard J, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One 2014;9:e115060. [Crossref] [PubMed]
- Wildes TM, Ruwe AP, Fournier C, et al. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J Geriatr Oncol 2013;4:227-34. [Crossref] [PubMed]
- Kiderlen M, de Glas NA, Bastiaannet E, et al. Impact of comorbidity on outcome of older breast cancer patients: a FOCUS cohort study. Breast Cancer Res Treat 2014;145:185-92. [Crossref] [PubMed]
- Ngeow J, Leong SS, Gaob F, et al. Impact of comorbidities on clinical outcomes in non-small cell lung cancer patients who are elderly and/or have poor performance status. Crit Rev Oncol Hematol 2010;76:53-60. [Crossref] [PubMed]
- Maestu I, Muñoz J, Gómez-Aldaraví L, et al. Assessment of functional status, symptoms and comorbidity in elderly patients with advanced non-small-cell lung cancer (NSCLC) treated with gemcitabine and vinorelbine. Clin Transl Oncol 2007;9:99-105. [Crossref] [PubMed]
- Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assess- ment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol 2005;23:6865-72. [Crossref] [PubMed]
- Arrieta O, Michel Ortega RM, Villanueva-Rodriguex G, et al. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer 2010;10:50. [Crossref] [PubMed]
- Blanc-Bisson C, Fonck M, Rainfray M, et al. Under nutrition in elderly patients with cancer: target for diagnosis and intervention. Crit Rev Oncol Hematol 2008;67:243-54. [Crossref] [PubMed]
- Puts MT, Santos B, Hardt J, et al. An up date on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol 2014;25:307-15. [Crossref] [PubMed]
- Vergnenegre A, Corre R, Lena H, et al. How old is "too old" for translational research? Transl Lung Cancer Res 2014;3:116-9. [PubMed]
- Vergnenegre A, Corre R, Lena H. Management of elderly patients. Transl Lung Cancer Res 2013;2:200-7. [PubMed]


