|
Original Article
Prevalence of chronic obstructive pulmonary disease among stable chronic disease subjects in primary care in Trinidad, West Indies
Peterson Thorington1, Maria Rios1, Gina Avila1, Josia Henry1, C. Haynes1, Lexley M Pinto Pereira2, Terence AR Seemungal1
1Department of Clinical Medicine Sciences, The University of the West Indies, Trinidad and Tobago; 2Department of Paraclinical Sciences, The University of the West Indies, Trinidad and Tobago
Corresponding to: Terence Seemungal, MD. Department of Clinical Medical Sciences c/o, The General Hospital, Charlotte Street, Port of Spain. Tel: 868-623-4030; Fax: 868-663-4332. Email: terence.seemungal@sta.uwi.edu.
|
|
Abstract
The prevalence of COPD in the Caribbean is uncertain. Spirometric indices were assessed at chronic disease clinics in 353 subjects (African, 66; East Indian, 198; 109 male), mean age 56.51 years (non-COPD) vs 59.30 years (COPD). 77 (21.8%) patients had COPD. 33.3% of COPD subjects had chronic cough vs 19.7% of subjects without COPD. A history of at least one chest infection was related to low FEV1 (P=0.005). In subjects presenting with vascular disease the FVC was reduced when compared to other subjects. Prevalence of COPD is 21.8%. A history of chest infections is related to decreased FEV1%.
J Thorac Dis 2011;3:177-182. DOI: 10.3978/j.issn.2072-1439.2011.03.03
|
|
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of death worldwide ( 1). It is a disease that represents a major health problem as it leads to increased disability in subjects, with increased health burden on the society ( 2). COPD is a progressive disease associated with airway inflammation and is characterized by airflow limitation that is not fully reversible and which is measured by the ratio of the forced expiratory volume in one second (FEV1) to the forced vital capacity (FVC) ( 3). The severity of COPD is ascertained by decrease in FEV1% predicted for age and height (FEV1%) ( 3). Accurate information on disease prevalence of COPD is important to understand its impact on disability, quality of life and healthcare costs, and to inform on public health planning ( 4). Baseline prevalence rates also allow epidemiological analysis to monitor trends, and determine the success or failure of control efforts. Despite the worldwide high prevalence of COPD, studies of airway diseases in Caribbean territories have concentrated on asthma. The single study which looked at the prevalence of COPD in Trinidad using measurements of lung function was a 2004 study of 720 acute medical admissions at the General Hospital, Port of Spain which showed that COPD was present in 21% of acute medical admissions. The study also showed that subjects with a low FEV1 were more likely to have cardiovascular disease ( 5). In a follow-up study to this, Cho Fook Lun et al. showed that subjects with COPD are more likely to have higher levels of CRP and homocysteine, (which are biochemical markers of inflammation and cardiac risk), compared to age and sex-matched controls ( 6). Based on these findings we hypothesized that COPD is highly prevalent in subjects with chronic disease in Trinidad. The setting was in chronic disease clinics in the primary health centers in Trinidad. We described relationships between lung function variables and explanatory variables in the population studied.
|
|
Methodology
Ethical permission for the study was obtained from the appropriate regional Health Authorities and from the Ethics Committee of the Faculty of Medical Sciences, University of the West Indies (St. Augustine Campus), prior to recruitment of subjects. All subjects gave informed written consent.
The study was conducted at five regional health centre chronic disease clinics at Arima, Chaguanas, Couva, Freeport and Marabella, all in Trinidad.
Inclusion and exclusion criteria
Subjects over the age of eighteen ( 18) years attending the chronic disease clinics at the aforementioned health centers during the
period June – August 2006 were sampled, as they presented.
Patients were excluded only if spirometry was contraindicated
according to ATS criteria ( 7). Additionally, some patients were
excluded if they were being treated with beta-blockers, upon the
request of their physicians. Dyspnoea was assessed using the Modified MRC Dyspnoea
Scale ( 8) and post-bronchodilator spirometric indices were
assessed according to the guidelines of the American Thoracic
Society (ATS) ( 7) by open-circuit testing. Data that was
included in the analysis satisfied the parameters of acceptability
and reproducibility ( 9). Subjects were classified into smokers
and never-smokers; and by number of cigarettes used per day
or grams of tobacco smoked per week. Patients were also asked
to recall the number of chest infections requiring antibiotics or
hospital admission within the past year. A patient was taken as having vascular disease if any one or
more of the following was stated in the medical notes: stroke,
ischaemic heart disease, myocardial infarction, hypertension,
congestive cardiac failure (with HTN and/or diabetes mellitus),
or cardiac arrhythmia due to ischaemic heart disease and/or
heart block ( 5). Data was expressed as mean (standard deviation, SD) where
normally distributed and otherwise as median (interquartile
range, IQR). Statistical significance was taken at the 5% level.
FEV1% predicted was normally distributed but other lung
function parameters were not; thus, bivariate relationships were
examined by Spearman’s correlations. Variables having significant
univariate relationship to FEV1% predicted at or less than the
10% level of significance were included in a backward stepwise
linear regression. Some variables known to have significant
relation to FEV1% from published data were also forced into
the multivariate analysis: BMI, age and gender. For this analysis,
skewed data were dichotomized about the median and binary
coded. Backward stepwise logistic regression was also used to
analyze multivariate relationships to the presence (or absence)
of vascular disease. SPSS version 12 for windows was used for
analysis.
|
|
Results
69 patients were excluded from the study as follows; (2.9%)
hemoptysis of unknown origin, (14.5%) unstable cardiac status,
(7.2%) recent eye, thoracic or abdominal surgery, (8.7%)
confused subjects, (1.4%) cannot sit up even with assistance,
(8.7%) severe chest and abdominal pain, (42.0%) beta blocker
on physician’s advice, (14.5%) left clinic before test was done.
A total of 353 subjects were included of which 66 were of African
ethnicity,198 were East Indians and 89 were classed as ‘other’.
There were 77(21.81%) patients with a spirometric diagnosis of
COPD, and these patients were older (P=0.05) with lower BMI
(P=0.08) than patients without COPD. There was no difference
in ethnicity between the two groups of patients ( Table1).
Comorbidity within the clinics
The major chronic diseases managed in the clinics were angina
(n= 53; 15%), cardiac failure (n=6, 1.7%), diabetes (n=159;
45%), HTN (n= 230, 65.2%). 48.7% of patients in the clinic had
one of these conditions and 33.1% had two. Further, as might be
expected in a clinic with 45% diabetics, 36% patients had a BMI
of more than 30 kg per square meter.
Smoking history and symptoms
75(27.2%) of the non- COPD patients were smokers whereas
30(39.0%) of the COPD patients were smokers, (P=0.045). The
non-COPD smokers had a smoking burden of 5.12 (16.4) pack
years while the COPD smokers had a burden of 8.26(23.4) pack
years.
Patients with COPD had significantly lower FEV1 and FEV1%
predicted but not FVC compared with the non-COPD group
( Table 2). As opposed to 54(19.7%) of the non- COPD patients
who had chronic cough, 25(33.3%) of the COPD patients
reported this symptom (P=0.01). 39(14.2%) of the non- COPD
patients complained of chronic sputum production, while
16(21.3%) of the COPD patients had cough with expectoration.
There was no difference between the two groups of patients on
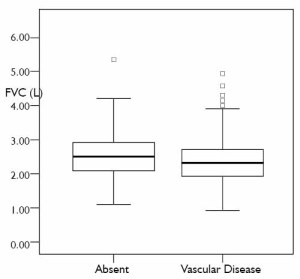
MRC dyspnoea grade. 248(70.25%) subjects had vascular disease. In subjects with
vascular disease the median FVC was lower [2.18 (1.76, 2.66)
L ] than those without vascular disease [2.40 (1.98, 2.92) L ]
( Fig 1). Of the 248 patients with vascular disease, 55 (22.18%)
had COPD. Several factors correlated with presence of vascular
disease: FEV1 (-0.150, 0.0074), FVC% (-0.137, 0.014), age
(0.205, ≤0.001), BMI (0.159, 0.004), but not FEV1% predicted
or FEV1/FVC (P>0.12 in both cases). When these correlated
variables were entered into a multivariate logistic regression with presence of vascular disease as an outcome variable FVC%
(-0.014, 0.061), Age (0.042, < 0.001), BMI (0.776, 0.003) were
found to be independently related to vascular disease. Subjects who were able to recall having at least one chest
infection were more likely to have a low FEV1 or FVC ( Table 3). Patients with at least one admission for a chest infection were
more likely to have a low FEV1, FEV1% or FVC %. Multivariate
linear regression with FEV1% as outcome variable revealed
independent relationships with high BMI, FVC%, history of at
least one chest infection and history of smoking ( Table 4).
| Table 1. Demographic data, past medical history, smoking history and MRC Dyspnoea grades for COPD and non-COPD patients |
| Variable |
Non-COPD patients n=276 |
COPD patients n=77 |
P value |
| Age/years mean (SD) |
56.51 (11.28) |
59.30 (10.93) |
0.052 |
| Body mass index mean (SD) |
29.19 (5.76) |
27.95 (5.54) |
0.088 |
| Height/metres mean (SD) |
1.61 (9.15) |
1.63 (10.50) |
0.149 |
| Gender n (%) |
|
|
0.083 |
| Male |
79 (28.6) |
30 (39.0) |
|
| Female |
197 (71.4) |
47 (61.0) |
|
| Ethnicity n (%) |
|
|
0.795 |
| East Indian |
154 (55.8) |
44 (57.1) |
|
| African |
54 (19.6) |
12 (15.6) |
|
| Other |
68 (24.6) |
21 (27.3) |
|
| At least one chest infection/lifetime n (%) |
73 (26.4) |
19 (24.7) |
0.754 |
| Chest admissions/last year n (%) |
5 (1.8) |
2 (2.6) |
0.662 |
| At least one hospital admission/ last year n (%) |
32 (11.6) |
10 (13.0) |
0.739 |
| Smokers n (%) |
75 (27.2) |
30 (39.0) |
0.045 |
| Pack years mean (SD) |
5.12 (16.4) |
8.26 (23.4) |
0.272 |
| Symptoms n (%) |
|
|
|
| Chronic cough |
54 (19.7) |
25 (33.3) |
0.012 |
| Chronic sputum |
39 (14.2) |
16 (21.3) |
0.135 |
| MRC Dyspnoea grade n (%) |
|
|
|
| MRC stage 0 or 1 |
227 (82.5) |
57 (74.0) |
0.094 |
| MRC stage 2, 3 or 4 |
48 (17.5) |
20 (26.0) |
|
| Table 2. Lung function parameters between COPD and non-COPD subjects |
| Variable |
Non-COPD patients n=276 |
COPD patients n=77 |
P value |
| Lung function parameters
median (IQR) |
|
|
|
| FEV1/L |
1.82 (1.47, 2.20) |
1.44 (1.10, 1.72) |
0.0010 |
| FEV1 % predicted |
67.60 (57.59, 76.73) |
52.31 (40.76, 61.21) |
0.0010 |
| FVC/L |
2.21 (1.80, 2.69) |
2.41 (1.86, 2.93) |
0.063 |
| FVC% predicted |
66.33 (57.72, 75.30) |
68.35 (58.87, 77.25) |
0.205 |
| FEV1/ FVC (%) |
81.19 (75.92, 86.87) |
61.75 (53.74, 66.73) |
0.000 |
| Table 3. Spearman’s correlations between lung function, chest admissions and lower respiratory tract infections and the number of subjects with at least one chest admission in the last year |
| Variable |
At least one lower respiratory tract
infection in lifetime |
At least one admission for a chest
condition in the past year |
| |
Rho |
P |
rho |
P |
| Chest hospital admissions in last year |
0.100 |
0.059 |
1.000** |
0.0010 |
| Best FEV1 |
-0.158** |
0.003 |
1.300* |
0.015 |
| FEV1% predicted |
-0.175** |
0.001 |
-0.176** |
0.001 |
| Best FVC |
-0.152** |
0.004 |
-0.096 |
0.071 |
| FVC% predicted |
-0.109* |
0.041 |
-0.137* |
0.010 |
| FEV1/FVC ratio |
-0.046 |
0.358 |
-0.053 |
0.0321 |
| **. Correlation is significant to the 0.01 level (2-tailed); *. Correlation is significant to the 0.05 level (2-tailed). |
| Table 4. Multivariate analyses using linear regression with FEV1 % predicted as the dependent variable. Only parameters with significant relationship with FEV1% are shown |
| |
Regression co-efficient |
P-value |
| Intercept |
10.82 |
<0.001 |
| BMI |
2.072 |
0.05 |
| At least one chest infection/ lifetime |
-3.406 |
0.005 |
| FVC % Predicted |
0.799 |
<0.001 |
| Smoker |
-2.827 |
0.016 |
|
|
Discussion
This is the first study to assess the prevalence of COPD in
patients with chronic disease in a West Indian setting. Patients
reporting a diagnosis of asthma were excluded from GOLD stage
classifications. COPD was more prevalent amongst females and
those with a history of smoking but only 39% of COPD patients
admitted to a history of smoking.
The prevalence of COPD was higher in women than men in
our study but this was because more women attended the health centers sampled though there is substantial evidence to suggest
that women may be predisposed to suffer adverse respiratory
consequences of tobacco smoke with greater impairment of
lung function and earlier COPD development ( 10). COPD
surveillance data from the United States has shown increased
mortality rate in females compared to males ( 11). It may be
argued however that women, having higher life expectancies,
may simply be living longer so as to succumb to the effects
of smoking. Additionally there is substantial data to suggest
that further study into gender differences in COPD should be
investigated as differences exist in the clinical manifestations of
COPD, as well as its gender prevalence ( 12-14). It should be noted that smoking may not be totally
responsible for the higher prevalence of COPD in females in our
study. Other known risk factors for the development of COPD
include air pollution, infections and occupational exposures.
Air pollution, particularly fine particulate indoor air pollution
from biomass fuels disproportionately affects women. Studies
have shown that women are more susceptible to the effects of
biological or organic dust exposure reflected by an increased
prevalence of respiratory symptoms and chronic bronchitis ( 15).
Exposure to occupational dust has been shown to be related
to higher COPD prevalence as well, especially in women ( 16).
The BOLD study found that Cape Town, South Africa had the highest prevalence of stage 2 or higher COPD as well as high levels of occupational dust exposure as well as smoking rates ( 17).
In our study, most patients with COPD were in stage II (53.2%),
a finding that may hint at the need for further investigation of
occupational exposure in the development of COPD in Trinidad.
Measures may be needed to decrease such exposures, promote
cleaner fuels, improved stoves, better home ventilation and
reduce toxic dust and fume exposures. Smoking is the major known environmental risk factor for
the development of COPD and as expected, it was found that
a higher proportion of patients with COPD were smokers
compared to the non-COPD patients. Consistent with this
was the finding that FEV1% was lower in smokers. Further as
previously found COPD patients smoked for a greater number
of pack years than non-COPD patients. A greater percentage
of COPD patients had chronic cough, linking smoking pack
years and development of these symptoms. This finding is
especially intriguing as one study has shown that cough may
be a better predictor of airflow limitation and when used to
preselect smokers for spirometry testing, the proportion with
an FEV1 less than 80% was increased. Thus increased emphasis
on respiratory symptoms in smokers may aid in the detection of
COPD by targeting higher risk patients ( 18). Studies have also
shown that the prevalence of undetected airflow limitation is
high among asymptomatic smokers, a finding that supports the
need for targeted screening of patients who present with chronic
cough, and dyspnoea ( 19). Comorbid conditions were very common in our study and
previous studies have confirmed that pulmonary function,
represented by FEV1, is an independent risk factor for Ischemic
Heart Disease mortality ( 20). In fact, 71.4% of COPD patients
in our study presented with cardiovascular disease, indicating
a major link with reduced lung function suggesting that
greater emphasis should be given to investigation of comorbid
conditions by West Indian physicians in the management of
COPD. Further, individuals with COPD are more likely to be at
risk of vascular events due to preexisting cardiovascular disease ( 21). We also obser ved that in patients presenting with
cardiovascular conditions, FVC values were significantly lower
than in patients without vascular disease. It has been postulated
that diminished respiratory function as measured by FVC is
associated with increased risk of cardiovascular mortality but this
is largely unexplained. However previous studies by Friedman
et al ( 22) suggest that clinical research investigating predictive
value of FVC is necessary, in line with our findings. Our study has limitations. The cross-sectional design limits
our ability to describe progression of the stages of COPD. In
patient recruitment, our study focused on outpatient clinics
so that our sample did not represent those who do not attend
these health centers and it is likely that there was a gender bias in
sampling because of this. The non-COPD patients had reduced FEV1 and FVC as estimated from that predicted for age and
height and gender. This is not unexpected as the patients were
recruited from the chronic disease clinics of the primary care
system. Diabetes, obesity and cardiac failure are associated with
a restrictive ventilatory defect. Thus because of reduced FVC,
we may have missed some of the COPD patients within these
chronic disease clinics.
With COPD prevalence being found to be more or less
the same in chronic disease patients as in the acute setting of
the Port-of-Spain hospital study (21%) ( 5), this study should
prompt further investigation into disease prevalence and its
morbidity in the general population. Additionally, it is hoped
that the new unearthing of data relating lung function to
chest infections in our patients, will lead to greater emphasis
on spirometry in our patients and thus to more appropriate
management of airways diseases.
|
|
References
- Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet
2003;362:1053–61.[LinkOut]
- Ferrer M, Alonso J, Morera J, Marrades RM, Khalaf A, Aguar MC, et al.
Chronic obstructive pulmonary disease stage and health-related quality of
life. The Quality of Life of Chronic Obstructive Pulmonary Disease Study
Group. Ann Intern Med 1997;127:1072–9.[LinkOut]
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy
for the diagnosis, management, and prevention of chronic obstructive
pulmonary disease. NHLBI/WHO Global Initiative for Chronic
Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit
Care Med 2001;163:1256-76.[LinkOut]
- Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, et al.
The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and
design. COPD 2005;2:277–83.[LinkOut]
- Seemungal T, Harrinarine R, Rios M, Abiraj V, Ali A, Lacki N, et al.
Obstructive lung disease in acute medical patients. West Indian Med J
2008;57:7-13.[LinkOut]
- Seemungal TA, Lun JC, Davis G, Neblett C, Chinyepi N, Dookhan C, et al.
Plasma homocysteine is elevated in COPD patients and is related to COPD
severity. Int J Chron Obstruct Pulmon Dis 2007;2:313-21.[LinkOut]
- American Association for Respiratory Care. AARC clinical practice
guideline to spirometry, 1996 update. Respir Care 1996;41:629-36.[LinkOut]
- MRC Committee on Aetiology of Chronic Bronchitis. Standardised
questionnaires on respiratory symptoms. BMJ 1960;2:1665.[LinkOut]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al.
Standardisation of spirometry. Eur Respir J 2005;26:319-38.[LinkOut]
- Chapman KR. Chronic obstructive pulmonary disease: are women more
susceptible than men? Clin Chest Med 2004;25:331-41.[LinkOut]
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic
obstructive pulmonary disease surveillance--United States, 1971-2000.
MMWR Surveill Summ 2002;51:1-16.[LinkOut]
- Tanoue LT. Cigarette smoking and women’s respiratory health. Clin Chest Med 2000;21:47-65.[LinkOut]
- Soriano JB, Maier WC, Egger P, Visick G, Thakrar B, Sykes J, et al. Recent
trends in physician diagnosed COPD in women and men in the UK.
Thorax 2000;55:789-94.[LinkOut]
- Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD.
Chest 2001;119:1691-5.[LinkOut]
- Ran PX, Wang C, Yao WZ, Chen P, Kang J, Huang SG, et al. The risk factors
for chronic obstructive pulmonary disease in females in Chinese rural areas.
Zhonghua Nei Ke Za Zhi 2006;45:974–9. Chinese.[LinkOut]
- Matheson MC, Benke G, Raven J, Sim MR, Kromhout H, Vermeulen R,
et al. Biological dust exposure in the workplace is a risk factor for chronic
obstructive pulmonary disease. Thorax 2005;60:645–51.[LinkOut]
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino
DM, et al. International variation in the prevalence of COPD (the BOLD
Study): a population-based prevalence study. Lancet 2007;370:741–50.[LinkOut]
- Van Schayck CP, Loozen JM, Wagena E, Akkermans RP, Wesseling
GJ. Detecting patients at a high risk of developing chronic obstructive
pulmonary disease in general practice: cross sectional case finding study.
BMJ 2002;324:1370.[LinkOut]
- Khan A, Shabbir K, Ansari JK, Zia N. Comparison of forced expiratory
volume in one second (FEV1) among asymptomatic smokers and nonsmokers.
J Pak Med Assoc 2010;60:209-13.[LinkOut]
- Schünemann HJ, Dorn J, Grant BJ, Winkelstein W Jr, Trevisan M.
Pulmonary function is a long-term predictor of mortality in the general
population: 29-year follow-up of the Buffalo Health Study. Chest
2000;118:656-64.[LinkOut]
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary
disease at increased risk of cardiovascular diseases? The potential role
of systemic inflammation in chronic obstructive pulmonary disease.
Circulation 2003;107:1514-9.[LinkOut]
- Friedman GD, Klatsky AL, Siegelaub AB. Lung function and risk
of myocardial infarction and sudden cardiac death. N Engl J Med
1976;294:1071-5.[LinkOut]
Cite this article as: Thorington P, Rios M, Avila G, Henry J, Haynes C, Pinto Pereira LM, Seemungal T. Prevalence of chronic obstructive pulmonary disease among stable chronic disease subjects in primary care in Trinidad, West Indies. J Thorac Dis 2011;3(3):177-182. doi: 10.3978/j.issn.2072-1439.2011.03.03
|