Circulating tumor cells in lung cancer: cluster circulating tumor cells as hybrid epithelial-mesenchymal transition/mesenchymal-epithelial transition (E/M)
Metastasis is the most frequent cause of death among lung cancer patients (1), as such it is crucial to understand the mechanism of cancer metastasis to control the disease. Almost all metastases rise from isolated tumor cells (ITCs) which are dislodged from the original cancer lesion. Among patients with lung cancer, the regions where ITCs, which are reported as prognostic indicator, are detected vary in the pleural space (2), the lymph nodes (3), the parenchyma of the lung (4), the surgical margin of the lung (5), the bone marrow (6), the circulating peripheral blood (7) and so on. The circulating tumor cells (CTCs), which are one of the ITCs, is suitable for clinical-pathological investigation because the grade of intervention to extract CTCs is very little. Like as other ITCs, CTCs are dislodged from the original cancer lesion so that the CTCs are speculated to keep the same molecular characteristics as the original cancer lesion. As the molecular characteristics of the lung cancer cell attributes to tumor histology (8,9) and the status of epithelial-mesenchymal transition (EMT) of cancer cells in the cancer lesion (10-12), the same attribution of the molecular characteristics to CTCs is speculated.
Based on above research question, Lindsay et al., among patients with lung cancer, investigated CTCs extracted using CellSearch® method, which extracts EpCAM positive (+) CD45 negative (−) single cells from the peripheral circulating blood, and revealed (13) that (I) the occurrence of CTC >5 was an indicator of poor prognosis; (II) the EMT status of CTC by way of vimentin was not an indicator of prognosis and (III) the positive relationship between vimentin status and molecular status was shown in the status of EGFR but in KRAS nor in ALK. Since the sub classification according to molecular status associates with pathological diagnoses (8,9) and the prognosis of patients (14), it is speculated that the molecular sub classification also associates with the EMT status of CTCs. However, the results from the Lindsay report are aside. The reason why the results fell in as shown in Lindsay report is that the target CTC selected was EpCAM (+) single CTC. If the Lindsay study employed another CTC selection method which can extract not only single CTCs but also cluster CTCs, the results might be different. This is because the EMT status of CTC changes time by time and is different between single CTC and cluster CTCs (15) (Figure 1).
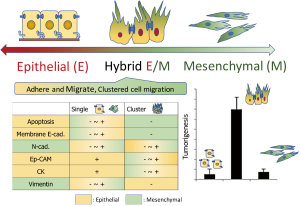
The CTCs, which is one aspect (“snap-shot”) of metastasis and dislodged from the original cancer lesion, varies in single CTC and cluster CTCs. The status of single or cluster depends on the EMT status which attribute to the potential of tumorigenesis, i.e., cluster CTCs pose greater potential of metastasis as 50 times as single CTC (16) (Figure 1). In days when the concept of EMT had been on developing, it is reported that the cluster cancer cells pose greater potential of cancer stem cell than single cancer cell (17,18). This may be because, as shown in Figure 1, the cluster cancer cells associate with hybrid (transitional) EMT/mesenchymal-epithelial transition (E/M) (15). The hybrid E/M CTCs are dislodged from the cancer lesion with cluster morphology posing abilities of (I) adherence, (II) migration, (III) anti-anoikis and (IV) tumor formation (19). In practice, among lung cancer patients who underwent surgery the detection of cluster CTCs is reported as an indicator of poor prognosis compared to single CTC alone or no detection of CTC (20,21). This phenomenon mentions the cruciality to distinguish cluster CTCs from single CTC. In addition, the pattern of expression of mesenchymal marker is revealed to be deferent at single CTC from at cluster CTCs (22) (Figure 1), above all vimentin expression occurred more frequently at single CTC than at cluster CTCs, as such more further insight is needed into the clinical implication of EMT markers at CTCs.
As mentioning so far, the tumorigenesis of CTC associates with morphological characteristics such as single or cluster which is a surrogate of high malignancy. The high potential tumorigenesis of cluster CTCs attributes to the status of hybrid E/M. In the study presented by Lindsay et al. (13) employed the CellSearch® as the method of CTC extraction. Because this equipment catches EpCAM positive CTC via magnetic antibody, the method is not good at catching cluster CTCs especially huge cluster CTCs (23). The representative commercially available CTC detection method are listed in Table 1 based on the ability to extract cluster CTCs (24,25). The methods via gravity for pulmonary vein (PV) blood or the method of size selection for peripheral blood may be recommended to extract cluster CTCs posing the status of hybrid E/M CTC which is crucial to control cancer metastasis.

Full table
The summary is follows: (I) CTCs are the “seeds” of metastasis; (II) the cluster CTCs, in comparison to single CTC, pose high potential to make metastasis; (III) the pattern of EMT marker appearance is different between single CTC and cluster CTCs which frequently express vimentin negative; (4) cluster CTCs are hybrid E/M with higher potential of (i) adherence, (ii) migration, (iii) anti-anoikis and (iv) tumor formation compered to single CTC and (v) detection method of CTC varies thus the ability to extract cluster CTCs is different each other and size selection method is recommended for peripheral circulating blood to extract cluster CTCs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Sawabata N, Asamura H, Goya T, et al. Japanese Lung Cancer Registry Study: first prospective enrollment of a large number of surgical and nonsurgical cases in 2002. J Thorac Oncol 2010;5:1369-75. [Crossref] [PubMed]
- Wang CM, Ling ZG, Wu YB, et al. Prognostic value of pleural lavage cytology in patients with lung cancer resection: an updated meta-analysis. PloS One 2016;11:e0157518. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Sawabata N, Funaki S, Shintani Y, et al. Lung excision of non-small-cell lung cancer leaves cancer cells in residual lobe: cytological detection using pulmonary vein blood. Interact Cardiovasc Thorac Surg 2016;22:131-5. [Crossref] [PubMed]
- Sawabata N, Maeda H, Matsumura A, et al. Clinical implications of the margin cytology findings and margin/tumor size ratio in patients who underwent pulmonary excision for peripheral non-small cell lung cancer. Surg Today 2012;42:238-44. [Crossref] [PubMed]
- Pantel K, Izbicki J, Passlick B, et al. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet 1996;347:649-53. [Crossref] [PubMed]
- Zeng C, Fan W, Zhang N, et al. Diagnostic performance of circulating tumor cells in lung cancer: a systematic review and meta-analysis. Int J Clin Exp Med 2017;10:1805-15.
- Ren S, Su C, Wang Z, et al. Epithelial phenotype as a predictive marker for response to EGFR-TKIs in non-small cell lung cancer patients with wild-type EGFR. Int J Cancer 2014;135:2962-71. [Crossref] [PubMed]
- Dong YJ, Cai YR, Zhou LJ, et al. Association between the histological subtype of lung adenocarcinoma, EGFR/KRAS mutation status and the ALK rearrangement according to the novel IASLC/ATS/ERS classification. Oncol Lett 2016;11:2552-8. [PubMed]
- Shao DD, Xue W, Krall EB, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 2014;158:171-84. [Crossref] [PubMed]
- Voena C, Varesio LM, Zhang L, et al. Oncogenic ALK regulates EMT in non-small cell lung carcinoma through repression of the epithelial splicing regulatory protein 1. Oncotarget 2016;7:33316. [Crossref] [PubMed]
- Ilie M, Szafer-Glusman E, Hofman V, et al. Expression of MET in circulating tumor cells correlates with expression in tumor tissue from advanced-stage lung cancer patients. Oncotarget 2017;8:26112. [PubMed]
- Lindsay CR, Faugeroux V, Michiels S, et al. A prospective examination of circulating tumor cell profiles in non-small-cell lung cancer molecular subgroups. Ann Oncol 2017;28:1523-31. [Crossref] [PubMed]
- Murakami S, Ito H, Tsubokawa N, et al. Prognostic value of the new IASLC/ATS/ERS classification of clinical stage IA lung adenocarcinoma. Lung Cancer 2015;90:199-204. [Crossref] [PubMed]
- Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013;339:580-4. [Crossref] [PubMed]
- Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014;158:1110-22. [Crossref] [PubMed]
- Kai K, D’Costa S, Yoon BI, et al. Characterization of side population cells in human malignant mesothelioma cell lines. Lung Cancer 2010;70:146-51. [Crossref] [PubMed]
- Yeunga TM, Gandhi SC, Wilding JL, et al. Cancer stem cells from colorec-tal cancer-derived cell lines. Proc Natl Acad Sci 2010;107:3722-7. [Crossref] [PubMed]
- Jolly MK, Tripathi SC, Jia D, et al. Stability of the hybrid epithelial/mesenchymal phenotype. Oncotarget 2016;7:27067. [Crossref] [PubMed]
- Funaki S, Sawabata N, Nakagiri T, et al. Novel approach for detection of isolated tumor cells in pulmonary veinusing negative selection method: morphological classification and clinical implications. Eur J Cardiothorac Surg 2011;40:322-7. [PubMed]
- Sawabata N, Funaki S, Hyakutake T, et al. Perioperative circulating tumor cells in surgical patients with non-small cell lung cancer: does surgical manipulation dislodge cancer cells thus allowing them to pass into the peripheral blood? Surg Today 2016;46:1402-9. [Crossref] [PubMed]
- Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastais biology in lung cancer. Am J Pathol 2011;178:989-96. [Crossref] [PubMed]
- Desitter I, Guerrouahe BS, Benali-Furet N, et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res 2011;31:427-41. [PubMed]
- Sawabata N. Cluster Circulating Tumor Cell Is Crucial in Surgically Resected Lung Cancer J Thorac Oncol 2017;12:e18-e19. [PubMed]
- Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer 2011;129:1651-60. [Crossref] [PubMed]


