Micropapillary lung adenocarcinoma and micrometastasis
Lung adenocarcinoma (ADC) is the most common type of lung cancer, and it has been subtyped by the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS) histologic classification. Invasive ADC has been classified into five predominant subtypes—lepidic, acinar, papillary, micropapillary (MIP), and solid (1). Semiquantitative assessment and reporting of each subtype in 5% increments is recommended to represent all subtype patterns. Several independent retrospective studies have already demonstrated the prognostic value of the new classification (2,3). Of patients with high-grade pattern tumors, the MIP and solid subtypes behave more aggressively and have a worse prognosis compared with low-grade pattern tumors with the lepidic subtype.
The MIP subtype is defined as tumor cells that grow in papillary tufts forming florets that lack fibrovascular cores (4). Several large studies with cohorts >300 patients have validated the negative prognostic significance of MIP-predominant tumors (2,3). Our group has demonstrated that the presence (≥5%) and increasing percentage of the MIP pattern is associated independently with an increased risk of local recurrence in patients who were treated with limited resection (e.g., wedge resection or segmentectomy) for small (≤2 cm) lung ADC (5). Additionally, Tsao et al. (6) suggested that patients with stage I–III ADC who have a MIP subtype may benefit from adjuvant chemotherapy and have exhibited improved disease-free survival (DFS) rates.
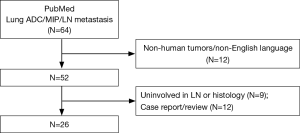
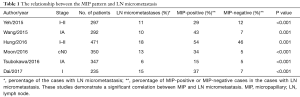
Lymph node (LN) metastasis is an important factor when determining treatment for non-small cell lung cancer (NSCLC), and a strong correlation between the MIP pattern and LN metastasis has been reported (Figure 1) (3,7). The MIP-predominant subtype, as well as a MIP component (≥5%), are both significantly associated with LN metastasis. The term “occult LN metastasis” is used to describe metastases that are not diagnosed by standard clinical and pathologic methods in node-negative LNs. LN micrometastasis, as a type of occult LN metastasis, is defined as isolated tumor cells or cellular clusters ≤0.2 mm in greatest dimension identified within LNs (4). Preoperative LN staging by computed tomography or 18F-fluorodeoxyglucose positron emission tomography fails to detect occult LN metastases and often exhibits a high false-negative rate (8). Even during histologic evaluation, it is difficult to identify this small focus of metastatic tumor cells on routine hematoxylin and eosin (H&E) slides. However, increasing evidence has shown that LN micrometastasis is predictive of poor prognosis in many malignancies including breast, colon, bladder, and lung cancer (9,10). Retrospective studies have reported that LN micrometastasis correlates with decreased overall survival (OS) and worse DFS in early-stage NSCLC (11-13). The MIP pattern is one of the most important factors reported to be related to a high risk of LN micrometastasis in early-stage lung cancer (Table 1).
Full table
Our group and Hung et al. (11,12) have investigated the relationship between primary tumor histologic patterns and occult LN metastasis in clinically N2-negative (cN0-1) lung ADC. Similar studies have been conducted by Wang et al. and Tsubokawa et al. in stage IA lung ADC and by Moon et al. in cN0 lung ADC (13-15). In these studies, the reported incidence rate of LN metastasis in early-stage lung cancer varied from 6% to 40%. While LN metastasis is present in 15% to 63% of patients with MIP-positive tumors, the metastatic rate of MIP-negative tumors is significantly lower. This suggests that the presence of the MIP pattern is significantly associated with occult LN metastasis.
Several methods have been used to detect LN micrometastasis including immunohistochemistry (IHC) and reverse transcriptase polymerase chain reaction (RT-PCR) (16,17). IHC was the standard and reliable method to detect LN micrometastasis in lung cancer. Several antibodies, such as cytokeratins (CAM 5.2 and AE1), Ber-EP4, and TTF-1, have been used to detect LN micrometastasis, and the majority of this research has demonstrated that LN micrometastasis detected by IHC is significantly associated with worse survival. RT-PCR for tumor-specific mRNA has been used to detect the carcinoembryonic antigen (CEA) in regional LNs of lung cancer. Even though RT-PCR is a more sensitive detection method, CEA is not specific to NSCLC, and, therefore, its clinical impact is insignificant.
Recently, Dai et al. (17) investigated the relationship between LN micrometastasis and histologic patterns in a cohort of 235 patients with stage I lung ADC. Immunohistochemical staining for cytokeratin (AE1/AE3) and thyroid transcription factor-1 (TTF-1) were used to identify LN micrometastasis. In their study, 23 (37%) cases with a MIP component had confirmed LN micrometastasis compared with only 12 (7%) cases without a MIP component that had confirmed LN micrometastasis. These LN tumor cells were positive for both AE1/AE3 and TTF-1. Dai et al. further indicated that MIP-positive/micrometastasis-positive patients had significantly worse survival compared with MIP-positive/micrometastasis-negative patients [recurrence-free survival (RFS), P=0.039; OS, P=0.002] and MIP-negative patients (RFS, P<0.001; OS, P<0.001). Moreover, in MIP-positive patients, the presence of LN micrometastasis correlated with a higher risk of locoregional recurrence (P=0.031) rather than distant recurrence (P=0.456). The strength of this study is the investigation of both LN micrometastasis and the MIP component on prognosis and recurrence pattern, which is a finding that has not been reported previously. This study, however, is limited by: (I) its retrospective nature carried out at a single center; (II) a small number of patients; and (III) a survival analysis that did not include lung cancer-specific survival, which is one of the most important survival parameters of lung cancer (18).
Although the MIP pattern has been shown to be related to poor prognosis, little is known about its biologic mechanism. Kamiya et al. (19) reported that the MIP component likely acquired anchorage-independent growth and a high potential for malignancy based on IHC for E-cadherin, beta-catenin, CD34, Ki-67, and laminin. Tsutsumida et al. (20) confirmed that high mucin 1 (MUC1) expression was present on the cell membranes of MIP pattern tumors. The expression of MUC1 is believed to be indicative of cell polarization inversion, which contributes to tumor invasion. Nagano et al. (21) discovered that expression of glucose transporter-1 (GLUT-1), a hypoxic marker, was higher in lung ADC tumors with a MIP component.
The most recently described method of invasion in NSCLC is spread through air spaces (STAS). Clinical research examining STAS has shown a positive association between this new pattern of invasion and presence of the MIP pattern and lymphatic invasion in the primary tumor (22,23). Jeong et al. (24) found no difference in endothelial cell proliferation in LN micrometastasis. This indicates that malignant cells may use the already present lymphatic vasculature to reach the LN. Vessel co-option of alveolar capillaries has been described as a method of survival implemented by isolated cancer cells in lung parenchyma (25). The MIP subtype, lymphatic invasion, and the possible co-option mechanism may provide some insight into the relationship between STAS around the primary tumor and LN metastasis.
In summary, the novel concept of the impact of the MIP component on LN micrometastasis and its consequent effect on prognosis need to be investigated in a large cohort of patients. Understanding the underlying biologic mechanisms may also yield clarification regarding the optimal surgical approach (lobectomy versus limited resection) and improve operative decision making for patients with lung ADC with a MIP component.
Acknowledgements
We thank Alex Torres of the MSK Thoracic Surgery Service for his editorial assistance.
Funding: The author’s research is supported by grants from the National Institutes of Health (P30 CA008748); the U.S. Department of Defense (BC132124 and LC160212); the Mesothelioma Applied Research Foundation; the Baker Street Foundation; the Derfner Foundation; the Joanne and John DallePezze Foundation; and the Mr. William H. Goodwin and Mrs. Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [Crossref] [PubMed]
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol 2015;10:1240-2. [Crossref] [PubMed]
- Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105:1212-20. [Crossref] [PubMed]
- Tsao MS, Marguet S, Le Teuff G, et al. Subtype Classification of Lung Adenocarcinoma Predicts Benefit From Adjuvant Chemotherapy in Patients Undergoing Complete Resection. J Clin Oncol 2015;33:3439-46. [Crossref] [PubMed]
- Lee G, Lee HY, Jeong JY, et al. Clinical impact of minimal micropapillary pattern in invasive lung adenocarcinoma: prognostic significance and survival outcomes. Am J Surg Pathol 2015;39:660-6. [Crossref] [PubMed]
- Kanzaki R, Higashiyama M, Fujiwara A, et al. Occult mediastinal lymph node metastasis in NSCLC patients diagnosed as clinical N0-1 by preoperative integrated FDG-PET/CT and CT: Risk factors, pattern, and histopathological study. Lung Cancer 2011;71:333-7. [Crossref] [PubMed]
- Akay CL, Albarracin C, Torstenson T, et al. Factors impacting the accuracy of intra-operative evaluation of sentinel lymph nodes in breast cancer. Breast J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Sloothaak DAM, van der Linden RLA, van de Velde CJH, et al. Prognostic implications of occult nodal tumour cells in stage I and II colon cancer: The correlation between micrometastasis and disease recurrence. Eur J Surg Oncol 2017;43:1456-62. [Crossref] [PubMed]
- Yeh YC, Nitadori J, Kadota K, et al. Micropapillary Histology Is Associated with Occult Lymph Node Metastasis (pN2) in Patients with Clinically N2-Negative (cN0/N1) Lung Adenocarcinoma. J Thorac Oncol 2013;8:S671-2.
- Hung JJ, Yeh YC, Jeng WJ, et al. Factors predicting occult lymph node metastasis in completely resected lung adenocarcinoma of 3 cm or smaller. Eur J Cardiothorac Surg 2016;50:329-36. [Crossref] [PubMed]
- Wang L, Jiang W, Zhan C, et al. Lymph node metastasis in clinical stage IA peripheral lung cancer. Lung Cancer 2015;90:41-6. [Crossref] [PubMed]
- Tsubokawa N, Mimae T, Sasada S, et al. Negative prognostic influence of micropapillary pattern in stage IA lung adenocarcinoma. Eur J Cardiothorac Surg 2016;49:293-9. [Crossref] [PubMed]
- Moon Y, Kim KS, Lee KY, et al. Clinicopathologic Factors Associated With Occult Lymph Node Metastasis in Patients With Clinically Diagnosed N0 Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1928-35. [Crossref] [PubMed]
- Rusch VW, Hawes D, Decker PA, et al. Occult metastases in lymph nodes predict survival in resectable non-small-cell lung cancer: report of the ACOSOG Z0040 trial. J Clin Oncol 2011;29:4313-9. [Crossref] [PubMed]
- Dai C, Xie H, Kadeer X, et al. Relationship of Lymph Node Micrometastasis and Micropapillary Component and Their Joint Influence on Prognosis of Patients With Stage I Lung Adenocarcinoma. Am J Surg Pathol 2017;41:1212-20. [Crossref] [PubMed]
- Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol 2017;35:281-90. [Crossref] [PubMed]
- Kamiya K, Hayashi Y, Douguchi J, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol 2008;21:992-1001. [Crossref] [PubMed]
- Tsutsumida H, Nomoto M, Goto M, et al. A micropapillary pattern is predictive of a poor prognosis in lung adenocarcinoma, and reduced surfactant apoprotein A expression in the micropapillary pattern is an excellent indicator of a poor prognosis. Mod Pathol 2007;20:638-47. [Crossref] [PubMed]
- Nagano T, Ishii G, Nagai K, et al. Structural and biological properties of a papillary component generating a micropapillary component in lung adenocarcinoma. Lung Cancer 2010;67:282-9. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Warth A, Muley T, Kossakowski CA, et al. Prognostic Impact of Intra-alveolar Tumor Spread in Pulmonary Adenocarcinoma. Am J Surg Pathol 2015;39:793-801. [Crossref] [PubMed]
- Jeong HS, Jones D, Liao S, et al. Investigation of the Lack of Angiogenesis in the Formation of Lymph Node Metastases. J Natl Cancer Inst 2015.107. [PubMed]
- Bridgeman VL, Vermeulen PB, Foo S, et al. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J Pathol 2017;241:362-74. [Crossref] [PubMed]


