No compensatory lung growth after resection in a one-year follow-up cohort of patients with lung cancer
Introduction
Various effects of pulmonary resection on lung growth have been reported in mammals. Compensatory lung growth has been well documented after pneumonectomy in various young and adult animals (1-5). Both parenchymal and nonparenchymal cells participate in this lung growth (1,6). In humans, the vital capacity and transfer factor for carbon monoxide (DLCO) are still slightly decreased 1 year after surgery, suggesting a long recovery after resection; however, these alterations are not direct signs of growth (7,8). Circulating growth factors seem to have a predominant impact on this compensatory effect. Insulin-like growth factor-1 (IGF-1) is a potential source of the mitogenic activity observed in the lung (9,10); however, the impact of growth factor concentrations on lung mass (mL) after lung resection has been poorly investigated. Lung growth following resection induces a secretion of growth factors stimulating tumor growth (11). A specific increase in the lung capillary blood volume (Vc) could be interpreted either as a hemodynamic adaptation of the lung after resection or as an abnormal stimulation of angiogenesis due to growth factors. Only one article has reported an increase in mL following right pneumonectomy in a young woman (12); however, no measurement of mL after lung resection has been reported in a cohort of patients.
The purpose of this study was to investigate compensatory lung growth after lung resection by measurements of mL, pulmonary capacity, pulmonary diffusion and circulating growth factors and to assess the putative role of the angiogenic process.
Methods
Patients
Patients were recruited consecutively when admitted for lung resection of known or suspected lung cancer. They were prospectively assessed over a two-year period. Inclusion factors were: non-small-cell lung cancer staged T1, T2 or T3A of TNM classification with or without cerebral or adrenal metastasis. Exclusion factors were: small-cell lung cancer, non-small-cell lung cancer staged 3B and 4, multiple metastasis, benign tumor, atypical resections, inoperable patient and neo-adjuvant treatment before surgery. This study was performed according to the declaration of Helsinki. All patients provided informed written consent for the study, which was approved by the Hospital Ethical Committee of Bordeaux (CCPRB A n 2005/58).
Protocol
Each patient had a routine full clinical assessment prior inclusion. Several measurements were performed at inclusion (M0), 3 months (M3) and 12 months (M12) after lung resection: IGF-1 and insulin-like growth factor binding protein-3 (IGFBP-3) plasma concentrations; mL of operated and non-operated lung via computerized tomography (CT); lung volumes; and CO and NO diffusion capacity (DLCO and DLNO), from which capillary lung volume Vc and DmCO the membrane conductance for CO were derived.
Pulmonary function
Pulmonary function tests included the measurements of total lung capacity (TLC), vital capacity (VC) by whole body plethysmography and DLCO and DLNO (Hypercompact +, Dinant Be). The single breath technique for the measurement of DLCO and DLNO has been described elsewhere (13). DmCO and Vc were calculated as described in previous studies (14,15). Diffusion tests were repeated twice at 5-min intervals. If a difference greater than 10% appeared, a third test was performed. The mean of two validated tests was calculated.
CT
The CT examinations were performed using a Siemens Somatom 16-slice scanner (Siemens medical system, Erlangen, Germany) with a volumetric acquisition covering both lungs and a collimation of 0.75 mm. Next, 1-mm-thick sections were reconstructed without gaps. Subjects were scanned in the supine position without an injection of IV contrast. The following parameters were employed: 100–120 KV and 60–80 mAs leading to a product dose-length varying between 100–150 mGy·cm depending on BMI. Attenuation values were expressed in HU. These units are related to density (d), d = 1 + (HU/1,000). Conventionally, water has a HU value of 0, and air has a value of ‒1,000. An average of 350 slices was required to cover the totality of both lungs. Images were photographed on hard copies with a 200, ‒1,000 HU window. The pulmoCT software (Siemens medical system, Erlangen, Germany) allowed the calculation of lung density and volume. The program is based on an algorithm that accurately delineates lung contour along the parietal and pleural surface from the clear-cut difference between lung density (‒1,000 HU) and pleural/mediastinal wall density (60 HU). The volume, density and mL of each lung were calculated separately. Tumor volume and density were defined by contouring the tumor by eye.
The slices were all selected at 5-mm, beginning at the top of the apex. The volume and mean HU of each slice in each lung were calculated separately. The mass of each slice was obtained by multiplying the mean density and the volume. The methodology used was similar to that described by Manier et al. (16). The entire lung was reconstructed from 30 to 40 contiguous slices. The mL was calculated as the sum of the mass of the slices. The weight of the tumor was excluded from the calculation of the lung weight. The hilar and perihilar lung regions were excluded from the region of interest and the central great vessels using the software.
Immunoradiometric assay of IGF-1 and IGFBP-3
All the samples were assayed by trained staff at the hospital laboratory. The serum concentrations of IGF-1 and IGFBP-3 were measured by immuno-radiometric assay using commercially available kits (Immunotech). The mean intra-assay coefficient of variation for the quality control serum samples was 5.6% for IGF-1 and <4.4% for IGFBP-3. The range of reliable measurement was 0–160 ng/mL for IGF-1 and 0.3–100 ng/mL for IGFBP-3.
Statistical analysis
Data are expressed as the mean values ± SD or as the median (25th; 75th percentiles) after assessing the normality of the distributions. Statistical analyses were performed using SAS/STAT Software v8.2. (SAS Institute Inc., Cary, NC, USA). Analysis of variance (ANOVA) was performed for comparisons of repeated measurement or between groups. Paired t-tests or Wilcoxon tests were applied whenever appropriate. Linear regressions were calculated using the least squares method. A P value <0.05 was considered significant.
Results
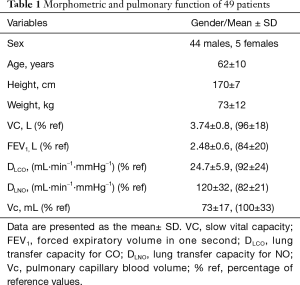
Patients (Table 1)
Full table
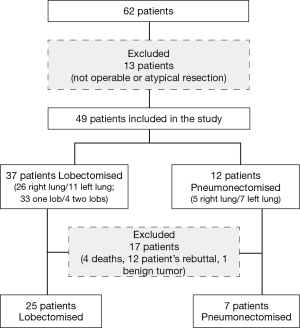
In total, 62 patients were recruited, and 13 were excluded from the study (Figure 1). The morphometric characteristics of the 49 patients are presented in Table 1. Among them, 12 patients underwent a pneumonectomy, and 37 patients had a lobectomy. Seventeen patients did not complete the protocol, 4 died, 12 gave up and 1 was excluded as having a benign tumor. Pre-operative treatment was performed in 5 patients. The postoperative TNM classification of the lungs was as follows: 18 stage 1; 13 stage 2; 17 stage 3; and 1 stage 4. The types of cancer cells included were as follows: 26 adenocarcinomas, 21 squamous cell carcinomas, and 1 neuroendocrine cancer. After surgery, adjuvant treatment was performed in 17 patients, among which 13 completed the study. The median mass (25th; 75th percentiles) of the tumor was lower in the group of lobectomies [24 g (4.3; 49)] compared with the pneumonectomies [171 g (7.6; 335)]. In total, 34% of the patients experienced lung cancer recurrence during the time of the study.
Lung masses
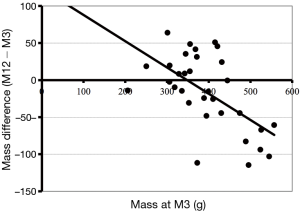
At inclusion, left and right mL did not differ regardless of the site of the tumor. Lobectomy induced an mL average decrease of 138 g of the lung (P<0.001); this mass was maintained between M3 and M12 (Table 2). The average mass of the non-operated lung did not change significantly between M0 and M12; however, the change of mass between M3 and M12 was inversely proportional to the mass at M3, this relationship was significant (P=0.0004) (Figure 2). In other words, the lightest lungs at M3 gained mass from M3 to M12 while conversely the heaviest lung could loss mass.

Full table
Of 32 patients, 13 received chemotherapy and radiotherapy after surgery. This group had a lower non-operated mL than the group without treatment at M3 (352.5 vs. 415 g, P=0.0017). This difference fade out at M12 as the mL increased in the group with chemotherapy (+20 g) as it decreased (‒31 g) in the group without chemotherapy.
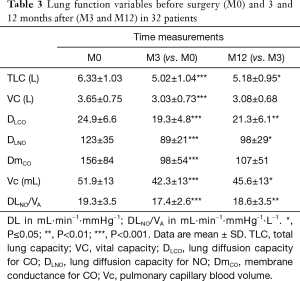
Lung function (Table 3)
Full table
The pulmonary functions of the patients at M0 were within the normal reference values (Table 1). Lung resection induced a significant decrease in TLC by 1.31±0.9 L. From M3 to M12, TLC increased by 0.17±0.4 L (P=0.03) while the change in VC was not significant. DLCO and DLNO values were greater at M12 compared with M3 (P≤0.01). The ratio of DLNO/VA decreased after resection and returned towards its initial value at M12. Both Vc and DmCO were slightly increased between M3 and M12. The increase was significant for Vc (P=0.05). The decrease in Vc from 51.9±13 to 42.3±13 mL after resection was partially restored 12 months after lung surgery to 45.6±13 mL.
IGF-1 and IGFBP-3 (Table 4)

Full table
At inclusion, IGF-1 and IGFBP-3 concentrations were 135±48 and 2.38±0.8 ng/L respectively. Both concentrations remained steady at M3. Then, from M3 to M12, IGF-1 concentration decreased from 146±57 to 116±48 ng/L (P<0.0001), and IGFBP-3 decreased from 2.38±0.8 to 2.25±0.7 ng/L (P=0.08).
Discussion
Lung mass and lung function were assessed during the one-year period after pulmonary resection in a cohort of patients receiving lung cancer operations. The main finding is that the non-operated and operated lungs had not grown 1 year after lung resection.
Compensatory lung growth after lung resection
Age dependency of lung growth
Immature dogs responded robustly to major lung resection (5). The adult/puppy comparison demonstrates an enhanced regenerative alveolar-capillary growth in the immature dog after pneumonectomy (17). Hsia et al. concluded that in adult dogs after left pneumonectomy, compensation by tissue growth is quite limited, and only adjustments occur in existing structures of the remaining lung (18). In the present study, patients were, on average, in the last fourth of the predicted life span for man. It is worth noting that the only increase in mL after pulmonary resection was observed in a 33-year-old woman (12).
Time dependency of lung growth
Three months after surgery mL changes were minimally detected because of the acute remodeling processes and the effects of post-surgical therapies. Inflammation, which increases mL and blood flow, can persist only a few months after lung resection (19,20). In the present cohort, the mL of the non-operated lung did not change significantly at M3, but individual values were scattered, suggesting that some patients exhibit a transient increase in their mL that is subsequently lost, as demonstrated by the negative correlation between the changes in mL between M3 and M12 and mass at M3.
The case reported by Butler et al. suggested that compensatory lung growth requires years (12), which is consistent with the fact that no increase in lung weight was observed in the present cohort during a one-year follow-up.
Surgery location dependency of lung growth
Some authors suggested that the compensatory lung growth depends on the volume of lung resected and the location of resection (21). The present data suggest that a relatively heavy non-operated lung loses mass between M3 and M12, whereas light lungs gained mass. These changes may not be due to growth but to water content associated with acute post-surgery processes.
Other factors altering lung growth
Compensatory lung growth hypothesized includes passive structural changes by an adjustment of existing structures in the remaining lung (3,5,6,17). These changed may include enlargement of alveolar spaces, thinning of the alveolo-capillary membrane, increase in capillary blood volume etc. Otherwise, lung growth response may be induced by growth factors. Several growth factors may be involved in this compensatory response among which IGF-1 that acts on development of various tissues by linking with IGFBP-3 in blood (9-11).
Lung function after lung resection
As changes in mL after months are likely related to lung growth, immediate changes in lung function are related to the mechanical adaptation of the lung to the resection without growth. Such was the case in this study in which the average mass of both non-operated and operated lungs did not change, whereas the function of the lungs was progressively altered. The increase in TLC is consistent with the results reported by Nagamatsu (8). As the mass of the lung did not increase concomitantly, these changes in lung volumes strengthen the hypothesis of a stretching effect of the chest through the remaining parenchyma.
The DLCO/DLNO method was used to calculate Vc and DmCO. It has been recently suggested that DLCO mainly depends on Vc, making this measurement a good marker of microcirculation (14) given that DLNO depends equally on Vc and Dm. Bolliger et al. observed that DLCO at rest was decreased 3 months after lobectomy or pneumonectomy, whereas DLCO/VA did not change (7). In the present study, Dm and Vc decreased from M0 to M3 by 33% and 20%, respectively. The predominance of the decrease in Dm and DLNO/VA suggest the induction of a slight interstitial edema. These alterations could also be due to early changes in pulmonary hemodynamics after resection. Tayama et al. reported a positive correlation between total pulmonary vascular resistance (PVR) and BNP plasma concentration a few days after lung resection (22).
Between M3 and M12, the diffusion variables increased, suggesting an adaptation of the capillary network of the lung to the conditions of lung resection. Just after the resection the increase in PVR would induce at constant cardiac blood flow a raise in vascular pressure and an increase in volume of the most compliant part of the circuit, i.e., in capillary blood volume. The increase in shear stress might also induce arterial vasodilation and a concomitant decrease in PVR (23). In the long term lung growth could be induced as suggested by experimental findings, morphometric analyses highlighting the compensatory growth of capillary volume and surface area one year after pneumonectomy in dogs (5). Capillary growth alone would have no measurable effect on the lung weight of an adult human, as the mass of blood in capillaries compared with the total mL is small; a 10% change in Vc would represent approximately 6 g in the present cohort. If no bronchial growth occurred, the increase in weight would remain non-significant. In the case study by Butler et al., a gain in lung weight was observed after several years; the alveolar and bronchiolar proliferation probably took more time than capillary proliferation (12).
Growth factor concentrations following lung resection
Changes in blood growth factor concentrations in human after lung resection has not been extensively studied. In the present study, IGF-1 and IGFBP-3 concentrations were altered 3 months after surgery with a marked decrease in IGF-1 (‒20%) observed between M3 and M12. These results were unexpected as IGF-1 and IGFPB-3 are known to induce epithelial proliferation and angiogenesis (24). Studies evaluating the changes in IGF-1 following lung surgery in animals provided differing results; however, most studies suggest an increase in IGF-1 (25,26). The IGF-1 decrease from M3 to M12 could be explained by several parameters, including aging and poor nutritional status (27). This status was not precisely evaluated in this study, and the duration of the follow-up was only 1 year. This decrease was more likely due to the lack of IGF-1 secretion after the removal of cancer cells (28).
Limitations and power of the study
A limitation of the study is the sample size. The lack of statistical power may explain the absence of significant differences in mL between M3 and M12. mL measurement implies many parameters that cause unavoidable variation. However biological markers have higher statistical power due to reduced measurement error. The absence of alteration of IGF-1 and IGFBP-3 concentrations between M0 and M3, followed by their decreases at M12 gives an argument against the stimulation of lung growth.
Another limitation is the duration of the follow-up. A longer duration would have increased the likelihood to observe a lung growth. However owing to the death rate of this population a longer duration would have need more patients which was out of our means.
Finally, among patients included in this study, some received post-operative treatment that could be suspected to slow down lung growth. However, in this sub-group, mL in contrast to the other, increased slightly from M3 to M12.
Conclusions
In conclusion, no compensatory lung growth was observed in a human cohort after lung resection during a one-year follow-up. No increase in the concentrations of IGF-1 and IGFBP-3 were observed. The slight capillary lung volume increase between M3 and M12 was likely due to minimal hemodynamic adaptation after resection. As the biological markers were not increased an angiogenic effect does not appear to be involved.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Hospital Ethical Committee of Bordeaux (CCPRB A n 2005/58) and written informed consent was obtained from all patients.
References
- Voswinckel R, Motejl V, Fehrenbach A, et al. Characterisation of post-pneumonectomy lung growth in adult mice. Eur Respir J 2004;24:524-32. [Crossref] [PubMed]
- Langston C, Sachdeva P, Cowan MJ, et al. Alveolar multiplication in the contralateral lung after unilateral pneumonectomy in the rabbit. Am Rev Respir Dis 1977;115:7-13. [PubMed]
- McBride JT. Lung volumes after an increase in lung distension in pneumonectomized ferrets. J Appl Physiol (1985) 1989;67:1418-21. [PubMed]
- Thurlbeck WM, Galaugher W, Mathers J. Adaptive response to pneumonectomy in puppies. Thorax 1981;36:424-7. [Crossref] [PubMed]
- Hsia CC, Herazo LF, Fryder-Doffey F, et al. Compensatory lung growth occurs in adult dogs after right pneumonectomy. J Clin Invest 1994;94:405-12. [Crossref] [PubMed]
- Cagle PT, Langston C, Goodman JC, et al. Autoradiographic assessment of the sequence of cellular proliferation in postpneumonectomy lung growth. Am J Respir Cell Mol Biol 1990;3:153-8. [Crossref] [PubMed]
- Bolliger CT, Jordan P, Solèr M, et al. Pulmonary function and exercise capacity after lung resection. Eur Respir J 1996;9:415-21. [Crossref] [PubMed]
- Nagamatsu Y, Maeshiro K, Kimura NY, et al. Long-term recovery of exercise capacity and pulmonary function after lobectomy. J Thorac Cardiovasc Surg 2007;134:1273-8. [Crossref] [PubMed]
- McAnulty RJ, Guerreiro D, Cambrey AD, et al. Growth factor activity in the lung during compensatory growth after pneumonectomy: evidence of a role for IGF-1. Eur Respir J 1992;5:739-47. [PubMed]
- Nobuhara KK, DiFiore JW, Ibla JC, et al. Insulin-like growth factor-I gene expression in three models of accelerated lung growth. J Pediatr Surg 1998;33:1057-60; discussion 1061. [Crossref] [PubMed]
- Vadgama JV, Wu Y, Datta G, et al. Plasma insulin-like growth factor-I and serum IGF-binding protein 3 can be associated with the progression of breast cancer, and predict the risk of recurrence and the probability of survival in African-American and Hispanic women. Oncology 1999;57:330-40. [Crossref] [PubMed]
- Butler JP, Loring SH, Patz S, et al. Evidence for adult lung growth in humans. N Engl J Med 2012;367:244-7. [Crossref] [PubMed]
- Glenet SN, de Bisschop CM, Dridi R, et al. Membrane conductance in trained and untrained subjects using either steady state or single breath measurements of NO transfer. Nitric Oxide 2006;15:199-208. [Crossref] [PubMed]
- Martinot JB, Mule M, de Bisschop C, et al. Lung membrane conductance and capillary volume derived from the NO and CO transfer in high-altitude newcomers. J Appl Physiol (1985) 2013;115:157-66. [PubMed]
- Guénard HJ, Martinot JB, Martin S, et al. In vivo estimates of NO and CO conductance for haemoglobin and for lung transfer in humans. Respir Physiol Neurobiol 2016;228:1-8. [Crossref] [PubMed]
- Manier G, Duclos M, Arsac L, et al. Distribution of lung density after strenuous, prolonged exercise. J Appl Physiol 1999;87:83-9. [PubMed]
- Takeda S, Hsia CC, Wagner E, et al. Compensatory alveolar growth normalizes gas-exchange function in immature dogs after pneumonectomy. J Appl Physiol (1985) 1999;86:1301-10. [PubMed]
- Hsia CC, Fryder-Doffey F, Stalder-Nayarro V, et al. Structural changes underlying compensatory increase of diffusing capacity after left pneumonectomy in adult dogs. J Clin Invest 1993;92:758-64. [Crossref] [PubMed]
- Toda H, Murata A, Tanaka N, et al. Changes in serum granulocyte colony-stimulating factor (G-CSF) and interleukin 6 (IL-6) after surgical intervention. Res Commun Mol Pathol Pharmacol 1995;87:275-86. [PubMed]
- Uehara T, Yano T, Kuninaka S, et al. The influence of enhanced postoperative inflammation by the intrapleural administration of streptococcal preparation (OK-432) on the prognosis of completely resected non-small-cell lung cancer. J Surg Oncol 2000;75:51-4. [Crossref] [PubMed]
- Ravikumar P, Yilmaz C, Dane DM, et al. Defining a stimuli-response relationship in compensatory lung growth following major resection. J Appl Physiol (1985) 2013;116:816-24. [PubMed]
- Tayama K, Takamori S, Mitsuoka M, et al. Natriuretic peptides after pulmonary resection. Ann Thorac Surg 2002;73:1582-6. [Crossref] [PubMed]
- Dane DM, Yilmaz C, Gyawali D, et al. Perfusion-related stimuli for compensatory lung growth following pneumonectomy. J Appl Physiol (1985) 2016;121:312-23. [PubMed]
- Franklin SL, Ferry RJ Jr, Cohen P. Rapid insulin-like growth factor (IGF)-independent effects of IGF binding protein-3 on endothelial cell survival. J Clin Endocrinol Metab 2003;88:900-7. [Crossref] [PubMed]
- Al-Sahaf O, Wang JH, Browne TJ, et al. Surgical injury enhances the expression of genes that mediate breast cancer metastasis to the lung. Ann Surg 2010;252:1037-43. [Crossref] [PubMed]
- Price WA, Moats-Staats BM, Sekhon HS, et al. Expression of the insulin-like growth factor system in postpneumonectomy lung growth. Exp Lung Res 1998;24:203-17. [Crossref] [PubMed]
- Wolk A. The growth hormone and insulin-like growth factor I axis, and cancer. Lancet 2004;363:1336-7. [Crossref] [PubMed]
- Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004;363:1346-53. [Crossref] [PubMed]