Advancements in mechanical circulatory support for patients in acute and chronic heart failure
Introduction
Cardiogenic shock (CS), defined as acute cardiac hemodynamic instability and end organ hypoperfusion resultant from a primary cardiac disorder, continues to have high mortality and morbidity despite advances in pharmacological, mechanical, and reperfusion approaches to treatment (1). The etiology of CS varies with the most common etiology (~75%) being secondary to an acute coronary syndrome (ACS). Other causes include acute exacerbation of chronic heart failure (HF) (10–15%), valvular and other mechanical causes (5–10%), stress-induced cardiomyopathy (Tako-Tsubo; 1–5%), and myocarditis (1–5%) (2). Despite routine use of reperfusion therapy, ACS complicated by CS continues to have an impressive short term mortality of 35% (3). Initial treatment of CS from severe acute HF includes oxygen, diuretics, vasodilators, non-invasive ventilation, and inotropes. When CS is refractory to medical therapy, patients should be evaluated for percutaneous mechanical circulatory support (MCS) candidacy by a multi-disciplinary heart care team consisting of intensivists, cardiologist, and cardiothoracic surgeons. The degree of hemodynamic support given by various MCS therapies can vary with the gamut of devices ranging from intra-aortic balloon pumps (IABPs), to percutaneous temporary ventricular assist devices (VAD) to extracorporeal membrane oxygenation (ECMO). These various devices can aid, restore or maintain appropriate tissue perfusion before the development of irreversible end-organ damage. Some of the devices can provide either uni- or bi-ventricular support with the added benefit of respiratory support when combined with an oxygenator. Undoubtedly, technology has improved patient survival to recovery from CS, but in patients whom cardiac recovery does not occur, acute MCS can be effectively utilized as a bridge to long-term MCS devices and/or heart transplantation.
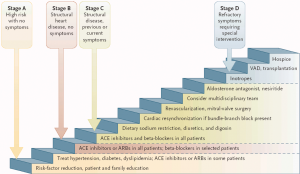
Treatment options for American College of Cardiology (ACC) Stage D HF patients are limited to the use of continuous inotropes, LVAD implantation, heart transplantation, and ultimately hospice and palliative care (4) (Figure 1). Heart transplantation remains the gold standard treatment for advanced chronic HF, but a limited availability of transplant hearts for a growing population of end-stage HF patients has led to a greater role of left ventricular assist device (LVAD) support. In 2001, the REMATCH trial demonstrated a significant increase in one year survival of end-stage HF patients implanted with pulsatile HeartMate XVE LVAD (Abbott, Lake Bluff, IL, USA) vs. medical therapy alone (52% vs. 25%) (5). The next generation continuous axial flow HeartMate II (Abbott, Lake Bluff, IL, USA) pump demonstrated one year survival of 68% (6), and became the first pump fully approved device with the intent of destination therapy (DT) (7). The HeartMate II remains the most widely implanted LVAD with the most recent INTERMACS report (8) showing an increasing use of smaller, continuous centrifugal flow pumps designed to decrease barriers of LVAD implantation including pump failure, pump thrombosis, and stroke events. This new generation of pumps include the HeartWare HVAD (Medtronic Inc., Fridley, MN, USA), which was approved for bridge to transplant (BTT) in 2012 (9), the HeartMate III (Abbott, Lake Bluff, IL, USA) (10) and HeartAssist 5 (ReliantHeart, Inc., Houston, TX, USA) (11), which are currently in clinical trial. In patients with biventricular failure that are ineligible for LVAD implantation, further advancements in the total artificial heart (TAH) may allow for improved survival compared to medical therapy alone. Importantly, barriers to durable MCS remain, and future technological advances in LVAD designs are needed to enhance treatment outcomes of chronic HF.
In this review, we discuss the current state of acute and durable MCS, ongoing advances in LVADs and TAH devices, improved methods of durable MCS implantation and patient selection, and future MCS developments in this dynamic field that may allow for optimization of HF treatment.
Acute MCS
IABP
IABPs are the most widely used MCS device since its introduction in the 1960s, but recent studies have questioned its potential benefit. Theoretically, pump inflation at the onset of diastole increases coronary perfusion, and pump deflation at the end of diastole results in reduction in aortic end-diastolic and systolic pressures, allowing for decreased afterload, decreased cardiac work, decreased myocardial oxygen consumption and increased cardiac output. Prior to 2012, the American and European guidelines supported IABP use in CS with a class I recommendation. In the randomized prospective IABP-SHOCK II trial, IABP was not found to be associated with reduction in 30 day mortality in patients with CS complicating ACS (12) or 12 month all-cause mortality (13). Given these trials and potential adverse events of IABP including limb ischemia, bleeding, thrombocytopenia, infection, and aortic dissection, IABP was downgraded to class III (harm) by the European Society of Cardiology, advising against the use of IABP in CS patients (14). However, AHA/ACC guidelines recommend IABP as class IIa in CS (15), and the most recent Society for Cardiovascular Angiography and Interventions expert consensus on PCI without on-site cardiac surgery maintain IABP support during transport of unstable patients a requirement (16). Given the controversy surrounding its benefit, the use of IABP is decreasing (17), and its future role in the treatment of CS may continue to decline as superior devices such as ECMO, percutaneous MCS, and isolated RV support become more commercially available with data arising from clinical trials and clinical experience.
Percutaneous MCS
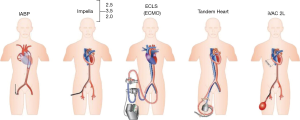
Current available percutaneous VADs offering temporary circulatory support include the non-pulsatile microaxial Impella 2.5, 5.0, and CP (Abiomed Europe, Aachen, Germany), and the Tandem Heart (Cardiac Assist, Inc., Pittsburgh, PA, USA). Other pumps under investigation include the pulsatile iVAC 2L (PulseCath BV, Arnhem, Netherlands), HeartMate percutaneous heart pump (Abbott, Lake Bluff, IL, USA) and the Impella RP (Abiomed Europe, Aachen, Germany), which is designed for univentricular RV support. The device specifications regarding mode of action, implant technique, cannula size, and flow are summarized (18) in Table 1 and Figure 2.
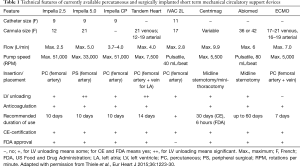
Full table
Data regarding percutaneous MCS devices in CS is limited. A meta-analysis of three randomized trials comparing percutaneous LVADs (two with TandemHeart and one with Impella 2.5) compared to IABP, percutaneous LVADs were associated with higher cardiac index, higher mean arterial pressure, lower pulmonary capillary wedge pressure (PCWP) but increased bleeding complications and no difference in 30-day mortality (19). In a recent randomized prospective trial of 48 patients, the Impella CP was not associated with decreased 30-day mortality in CS complicating ACS compared to IABP (20). Results from the USpella Registry show that Impella 2.5 use prior to PCI is associated with more complete revascularization and improved survival in the setting of refractory CS complicating ACS (21). Recently, the iVAC 2L pulsatile pump was shown to offer support in high risk PCI with 100% angiographic success in a prospective pilot study of 14 patients by den Uil et al. (22), but no trial results are currently available. An additional percutaneous LVAD under investigation has been the HeartMate percutaneous heart pump. A trial comparing this device to Impella support during high risk PCI began in 2014, but was paused in 2017 due to mechanical issues leading to pump stoppage (23).
Isolated RV failure has become more recognized in recent times, leading to development of devices specific for this cardiac dysfunction. The Impella RP is an intracardiac microaxial blood pump designed for the management of right ventricular failure (RVF) that can be inserted through the femoral vein. The prospective RECOVER RIGHT study showed that the safe, easily deployed, and reliable pump resulted in immediate hemodynamic benefit in patients with life-threatening RVF, leading to approval for use through a humanitarian device exemption (24).
Surgically placed temporary MCS
Surgically-implanted temporary MCS devices include the CentriMag (Abbott, Lake Bluff, IL, USA) and Abiomed (Abiomed Inc., Danvers, MA, USA) pumps. The CentriMag ventricular assist system can be used for univentricular or biventricular support for patients with CS, and was the first FDA-approved implantable VAD with biventricular capability (7,25). This system is typically implanted via median sternotomy. Recently, Takeda et al. (26) have developed a minimally invasive surgical approach combining ECMO with CentriMag VAD for short term CS treatment. The major advantage of this system in addition to full hemodynamic support is the ability to add an oxygenator to the right side of the configuration, thus providing complete right, left, and pulmonary support. This system showed non-inferior 30 day and overall 1 year survival vs. CentriMag BiVAD alone, but eliminated the need for cardiopulmonary bypass and reduced blood product utilization and bleeding events. The Abiomed ventricular assist system is another device with uni- or biventricular capabilities that is placed via sternotomy, but no randomized trials assessing its effectiveness are available.
ECMO
ECMO provides gas exchange as well as cardiac support and is used in patients suffering from respiratory failure, cardiac failure or both. Veno-venous ECMO is reserved for patients in isolated respiratory failure with no significant cardiac dysfunction, while veno-arterial ECMO (VA-ECMO) is considered in patients with cardiopulmonary collapse and is used to support patients in CS (27). In patients who fail to wean from cardiopulmonary bypass after open heart surgery, central VA-ECMO has been applied as bridge to recovery. However, in non-post-cardiotomy failure patients requiring urgent cardiac support, peripheral VA-ECMO through the femoral artery and vein is the most common approach. Peripheral VA-ECMO has limitations, including retrograde blood flow leading to LV afterload mismatch and inadequate LV decompression. To counteract this in addition to the traditional addition of a surgical LV vent, some centers utilize concurrent IABP (28) or Impella 2.5 (29) support to reduce the LV afterload and hence pulmonary edema. Recently, Naito et al. (30) describe a rotation speed modulation system that changes rotational speed in synchrony with the cardiac cycle of the native heart to offer the effects of VA-ECMO and IAPB in a single device, thus decreasing LV work load and afterload in CS patients, but these results from an in vivo goat model are yet to be applied in clinic.
The latest Extracorporeal Life Support Organization (ELSO) registry report shows ECMO use as well as the number of centers utilizing ECMO is increasing (31). Adults receiving ECMO for cardiac support from 1989–2015 show 41% survival to hospital discharge, with survival only increasing to 42% in the year 2015. Survival to discharge was dependent on VA-ECMO indication as CS (42%), cardiomyopathy (51%), myocarditis (65%), and congenital defect (37%). Absolute contraindications to VA-ECMO use include advanced age, chronic organ dysfunction (emphysema, cirrhosis, renal failure), compliance (financial, cognitive, psychiatric, or social limitations), and prolonged CPR without adequate tissue perfusion, while contraindications for anticoagulation, advanced age, and obesity are relative contraindications (ELSO Guidelines, 2013). Adverse events during the course of VA-ECMO are common (31), and it requires appropriately trained physicians and requisite healthcare infrastructure to prevent or mange these events. However, in the appropriate patient population, VA-ECMO is recommended as a useful tool that aids in acute HF treatment.
Durable MCS
Patients who are refractory to hemodynamic stability from CS and who continue to need mechanical support should be considered for transition to durable MCS. Temporary MCS restores hemodynamics and reverses end-organ dysfunction, but these patients have high residual risk with postoperative morbidity and mortality that parallels that of critical CS patients without temporary MCS (32). Although the use of temporary MCS is associated with considerably worse post-transplant survival compared to heart transplant without, overall post-transplant survival following temporary MCS is improving. While incidence for durable LVAD implantation in the CS patient population is increasing, the most common reason for durable LVAD implantation is chronic heart failure.
LVADs
LVADs were FDA-approved for BTT in 1998, and the landmark REMATCH trial that followed demonstrated a significant increase in one year survival in patients receiving pulsatile HeartMate I LVAD vs. medical therapy alone (52% vs. 25%) (5). However, due to device replacement in 21% of patients from limited durability, continuous-flow pumps were developed (33), and the HeartMate II LVAD was shown to have greater survival post implant compared to first generation pumps when implanted as BTT and DT (6,7,34). In these pumps, limitations including stroke, driveline infections, and RVF persisted, and an abrupt increase in pump thrombosis from 2011–2013 (35,36) highlighted the need for improvements in LVAD device design, perioperative management, and patient selection (37).
Recent innovations
To address the growing concern of pump thrombosis and stroke in chronic HF support, as well as the large proportion of patients implanted as DT and the need for devices that were more durable long-term, new LVAD devices with noncontact bearings via magnetic levitation have been developed to allow for rotation without friction or wear. Furthermore, these pumps are being designed to allow for remote monitoring capabilities to optimize flow to the body’s demand and give clinicians an additional non-invasive tool to monitor device function and optimize treatment. Currently, the only FDA-approved device employing this design is the HeartWare HVAD, while other devices under investigation include the HeartMate III and HeartAssist 5. Currently available devices as well as those undergoing trials are summarized in Table 2.
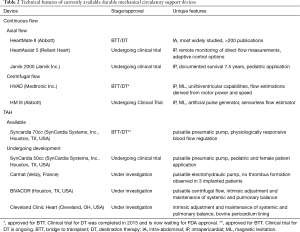
Full table
The HeartWare HVAD is a small intrapericardial centrifugal-flow pump approved for BTT in 2012. The ENDURANCE trial (9) was designed to evaluate the HVAD as DT and compared survival free from disabling stroke at two years between this device vs the HeartMate II control. Overall survival at two years did not differ significantly between the study group vs. control group (60.2% vs. 67.6%), while the study group had decreased device failure requiring replacement, increased stroke, and increased occurrence of sepsis. Given these results, non-inferiority of the device compared to the control was established.
The MOMENTUM 3 clinical trial is designed to evaluate the HeartMate III device for BTT and DT, comparing survival free of debilitating stroke or reoperation to replace the pump at 6 months and 2 years to the axial continuous flow HeartMate II pump (10). At six months, there were no significant between-group differences in rates of death or disabling stroke, but reoperation for pump malfunction was less frequent in the centrifugal-flow pump group (n=1, 0.7%) than axial flow (n=7, 7.7%). Secondary analysis of the trial showed the HeartMate III demonstrated greater freedom from hemocompatibility-related clinical adverse events vs. the HeartMate II at 6 months (38) and 1 year (39), and the trial is ongoing.
In addition to the potential benefits of a smaller, intrapericardial design and decreased device malfunction, new generations of LVADs may allow for noninvasive monitoring of pump function and flow. The HVAD allows for flow waveforms that provide information about HVAD function and patient hemodynamics. Recently, Grinstein et al. (40) describe that the slope of ventricular filling phase of HVAD waveforms is correlated with PCWP and can discriminate elevated versus normal or low PCWP. The HeartAssist 5 pump is currently undergoing clinical trial for use as BTT and takes this monitoring one step further by providing direct flow measurements through remote monitoring. In addition to flow data, the HeartAssist 5 tracks speed and electrical current usage by the pump motor, providing information about the volume of blood flow and its fluidity. Automated noninvasive tracking of pump function and flow offers an additional tool clinicians may use to help in the clinical assessment and management of patients.
Importance of patient selection and timing of LVAD implantation
New device designs and future innovations offer great potential to enhance treatment outcomes in advanced HF patients. Alternatively, appropriate patient selection and timing of LVAD implant offer additional routes toward maximization of therapy outcomes. The Randomized Evaluation of VAD InterVEntion before Inotropic Therapy (REVIVE-IT) pilot study began in 2009 to investigate the benefits of a more “aggressive” LVAD implant strategy vs. medical management in DT older patients with end stage HF. However, patient enrollment was delayed multiple times due to new studies showing increased incidence of LVAD pump thrombosis, and the trial was discontinued without ever beginning in 2015 (41,42). Similarly to the REVIVE-IT study, the Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients (ROADMAP) study sought to assess the outcomes of early LVAD implantation in less sick (INTERMACS 4-7) patients (43). Twelve month (80%±4% vs. 63%±5%; P=0.022) and two year (70%±5% vs. 41%±5%; P<0.001) (44) survival was greater in the LVAD vs. medical management group, as was health-related quality of life, while the LVAD group experienced more frequent adverse events and hospitalizations. These studies provide risk-benefit information to assist patient and physician decision making for elective LVAD therapy as a treatment for HF in non-inotrope dependent patients, but making an informed clinical choice between LVAD and medical therapy remains a challenge.
Improvements in perioperative management and implantation technique
The advancement of device technology and improved patient selection will continue to offer potential improvements in patient survival and freedom from adverse events (8). At the same time, careful perioperative management of LVAD patients and technique of LVAD implantation has been associated with better patient outcomes. The PREVENtion of HeartMate II Pump Thrombosis Through Clinical Management (PREVENT) (37) recommendations on implant technique, anti-coagulation and anti-platelet strategy, pump speed management, and blood pressure management were offered to address risk of early (<3 months) pump thrombosis with HeartMate II LVADs. Investigators found that full adherence to implant techniques, heparin bridging, and pump speeds >9,000 RPMs resulted in significantly lower risk of pump thrombosis (1.9% vs. 8.9%; P<0.01) and risk of suspected thrombosis, hemolysis, and ischemic stroke (5.7% vs. 17.7%; P<0.01) at 6 months. This stark decrease in early pump thrombosis associated with adherence to this set of recommendations supports the impact of surgical implant technique and clinical management practice in limiting pump thrombosis.
The smaller size of new pumps in combination with their intra-pericardial placement has led to the investigation of less invasive off-pump implantation techniques via mini-sternotomy and thoracotomy to limit surgical trauma and allow for early postoperative ambulation to optimize patient outcomes. This technique offers a surgery with potentially decreased bleeding, blood product transfusion, sternal wound infection, ventilation time, and intensive care unit stay. As the sternum is left largely intact, mechanical respiratory physiology is preserved, favoring downloading of the RV and decreased RVF (45). Additionally, it has been hypothesized that the largely intact pericardium may support the right ventricle and prevent distention (46). The first LVAD to be implanted off pump via thoracotomy and ministernotomy was the HeartWare HVAD (45-47), and similar techniques have since been described in the implantation of the CentriMag BiVAD system (48), and HeartMate 3 LVADs (49,50). Despite the advantages offered by a noninvasive and off-pump surgical technique, limitations remain (51,52). In surgeries requiring concomitant valve repair or replacement, this ministernotomy and thoracotomy approach is not feasible. Furthermore, the LVAD placement in the pleural space may create adhesions to the lung, phrenic nerve and/or diaphragm, complicating future operations. Given these risks and lack of clinical trials comparing the techniques, the advantage of minimally invasive off-pump surgical techniques over standard median sternotomy procedures remains unproven.
RVF and TAH developments
While RVF and right ventricular assist device (RVAD) use in LVAD patients have declined with better patient selectivity and utilization of intra-operative maneuvers (53) to reduce the strain on the right ventricle, RVF remains a serious complication associated with decreased survival (54). Univariate predictors of RVF such as DT intent of therapy, INTERMACS profile, preoperative hemodynamic profile, and baseline lab values have been proposed (55-57), and while a number of risk scores have been developed to predict RVF (55,58-60), appropriate selection and prediction of the RV prior to LVAD implementation remains imperfect (41). Current treatment options in patients with significant concomitant RV dysfunction to LVAD implantation include permanent LVAD with planned temporary right-sided RVAD. However, in patients with acute RVF post-LVAD implantation, as well as in patients with severe biventricular cardiomyopathy, complex congenital heart disease, failed transplantation, or acquired structural heart defects that have failed or remove the patient from conventional surgical treatment, TAH and off-label use of long-term RVAD offers a final alternative for survival while waiting for a donor transplant.
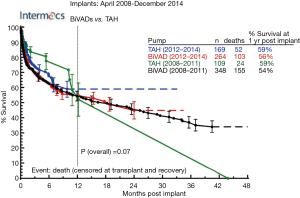
The idea of a TAH replacing the human heart has long been an intriguing challenge, with the first TAH implanted in a human in 1969 by Cooley et al. (61). Since this first TAH implantation, a number of devices and designs have been investigated (62), but none have been as successful as the Jarvik 7, later renamed CardioWest and now SynCardia TAH (SynCardia Systems, Inc., Houston, TX, USA), which relies on an external pulsatile pneumatic pump attached to the TAH through reinforced polyurethane drivelines. In 2004, CardioWest (now SynCardia 70cc) became the first and only TAH to get FDA approval for BTT (63,64), and clinical trial began in 2014 to evaluate the device for DT in biventricular patients ineligible for heart transplantation (65). Furthermore, the smaller SynCardia 50cc received FDA approval for an Investigational Device Exemption clinical study in 2015 to address the need for a smaller device in female and pediatric patients unable to receive the larger SynCardia 70cc device (66). Currently, INTERMACs registry data for biventricular HF patients receiving MCS as BTT shows one year mortality for SynCardia TAH is 59% vs. 56% for BiVADs (9). Despite the development of the SynCardia pumps, the pumps are still underutilized and only implanted in 50–80 patients per year (Figure 3) due to a ~59% post-implant one year survival (Figure 4) (9) but markedly decreased survival further past one year due to mechanical parts prone to malfunction, high incidence of thrombosis and hemoincompatibility (62). Other limitations include the need for a large and loud external motor, and dependency on an external energy supply.

The challenges to outcomes in patients implanted with the SynCardia TAH have led to continued developments of bioprosthetic TAHs to reduce anti-coagulation therapy and thrombosis-associated complications (67). The Carmat TAH (Carmat, Velizy, France) is a pulsatile electrohydraulic TAH that has been implanted in three patients, supporting one for 74 days before electronic failure, another for 270 days before mechanical device failure, and a third for 254 days (68,69). In all cases, autopsy did not detect any relevant thrombus formation within the bioprosthesis nor other organs. Two other TAH devices, the Cleveland Clinic continuous flow TAH (70) and the pulsatile BiVACOR TAH (71), have recently undergone in vivo calf studies, showing potential applicability to the clinical setting. These initial findings provide optimism of the use of bio-prosthetic materials in the construction of TAH going forward, but more study is required before these designs are prepared for widespread clinical use.
Future directions and technological advances of MCS
Over the last few decades, important improvements to MCS devices have allowed for improved patient survival and outcomes. Notwithstanding, the steady increase in patients requiring these devices, the large number being implanted as DT, and the continued occurrence of driveline infection, pump thrombosis, pump failure, cerebrovascular accidents, and RVF calls for further improvements for long-term sustainable and reliable LVAD support. Transcutaneous energy transfer (TET) has long been a potential option to eliminate driveline infections. TET would allow for the complete implantation of a device with a battery that can be recharged through intact skin of the patient, therefore removing the need for an external driveline. Although the LionHeart (72) (Arrow International, Inc., Research Triangle Park, NC, USA) and previously the AbioCor (73) (Abiomed, Inc., Danvers, MA, USA) devices utilized this technology, its impact in eliminating driveline infection has not been viewed as a great enough benefit at this point to overcome disadvantages in safety, economics, and administrative aspects (74).
As described earlier, HVAD flow waveforms potentially give physicians a non-invasive approach to analyze PCWP, while the new HeartAssist 5 LVAD offers direct flow measurement to precisely monitor blood flow and assist in the clinical assessment and management of patients supported by an LVAD (40). Importantly, future development of a smart LVAD pump that can adapt to changes in a patient’s physiology and respective perfusion demand may rely on similar pump measurements and analysis provided by these devices. Furthermore, new inflow cannulae designs may allow for decreased ventricular suction events while mitigating complications associated with hemolysis, as evidenced in an in vitro model by Pauls et al. (75). In vivo evaluation of a system combining a compliant outflow and inflow cannula with a sensor-based controller that altered left and right VAD speed based on pressure and flow showed promising results in preventing suction and pulmonary venous congestion in a sheep RVF model (76). Application and testing of these developments to LVADs and BiVADs in the human is a logical future step that may allow for decreased suction events, hemolysis, and RVD in patients.
To decrease thrombosis-associated complications and reduce anti-coagulation therapy, decellularized pericardium is being used in the construction of new generation TAH designs (67,70,71). Other approaches have been proposed, including titanium surfaces (77) and engineered gratings allowing for the migration and adhesion of endothelial cells leading to a fully confluent endothelial monolayer (74,77-79). The application of this type of material and these surfaces to future LVADs could further assist in pump hemocompatibility and allow for decreased pump thrombosis, cerebrovascular accidents, and bleeding.
Conclusions
CS refractory to medical therapy has high mortality and morbidity, and temporary MCS devices are seen as a last option in treatment of these patients. IABP remains the most widely used device for temporary MCS, but recent trial results have led to a decline in its use, while at the same time ECMO use and the number of centers utilizing ECMO is increasing. Percutaneous VAD pumps and surgically placed temporary MCS have been shown to be beneficial in observational trials, but randomized clinical trial data supporting their effectiveness is lacking. While these devices have increased HF patient survival to recovery, those in whom cardiac recovery does not occur require a heart transplantation or long-term MCS support via LVAD or TAH depending on whether or not biventricular failure is present. New LVAD devices are being designed to reduce the rate of adverse events. Undoubtedly with improvements in perioperative management, patient selection and timing of implant improvements to quality of life offered with these devices should continue to improve. However, complications including pump thrombosis, stroke, bleeding events, driveline infection, and RVF remain a major concern to wider adoption of device therapy. Future device innovations including remote monitoring of flow, new blood-contacting materials to promote pump hemocompatibility, and the use of TET to remove the need for a percutaneous driveline may aid in the reduction of these events and augment chronic HF treatment.
Acknowledgements
This work was supported in part by the American Association for Thoracic Surgery’s Summer Intern Scholarship.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Goldberg RJ, Makam RC, Yarzebski J, et al. Decade-Long Trends (2001-2011) in the Incidence and Hospital Death Rates Associated with the In-Hospital Development of Cardiogenic Shock after Acute Myocardial Infarction. Circ Cardiovasc Qual Outcomes 2016;9:117-25. [Crossref] [PubMed]
- Harjola VP, Lassus J, Sionis A, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail 2015;17:501-9. [Crossref] [PubMed]
- de Waha S, Jobs A, Eitel I, et al. Multivessel versus culprit lesion only percutaneous coronary intervention in cardiogenic shock complicating acute myocardial infarction: A systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care 2017.2048872617719640. [PubMed]
- Jessup M, Brozena S. Heart failure. N Engl J Med 2003;348:2007-18. [Crossref] [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [Crossref] [PubMed]
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885-96. [Crossref] [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015;34:1495-504. [Crossref] [PubMed]
- Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med 2017;376:451-60. [Crossref] [PubMed]
- Mehra MR, Naka Y, Uriel N, et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N Engl J Med 2017;376:440-50. [Crossref] [PubMed]
- Pektok E, Demirozu ZT, Arat N, et al. Remote monitoring of left ventricular assist device parameters after HeartAssist-5 implantation. Artif Organs 2013;37:820-5. [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet 2013;382:1638-45. [Crossref] [PubMed]
- Kolh P, Windecker S, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2014;46:517-92. [Crossref] [PubMed]
- O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:529-55. [Crossref] [PubMed]
- Dehmer GJ, Blankenship JC, Cilingiroglu M, et al. SCAI/ACC/AHA Expert Consensus Document: 2014 Update on Percutaneous Coronary Intervention Without On-Site Surgical Backup. Catheter Cardiovasc Interv 2014;84:169-87. [Crossref] [PubMed]
- Sandhu A, McCoy LA, Negi SI, et al. Use of mechanical circulatory support in patients undergoing percutaneous coronary intervention: insights from the National Cardiovascular Data Registry. Circulation 2015;132:1243-51. [Crossref] [PubMed]
- Thiele H, Ohman EM, Desch S, et al. Management of cardiogenic shock. Eur Heart J 2015;36:1223-30. [Crossref] [PubMed]
- Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J 2009;30:2102-8. [Crossref] [PubMed]
- Ouweneel DM, Eriksen E, Seyfarth M, et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump for Treating Cardiogenic Shock: Meta-Analysis. J Am Coll Cardiol 2017;69:358-60. [Crossref] [PubMed]
- O'Neill WW, Schreiber T, Wohns DH, et al. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol 2014;27:1-11. [Crossref] [PubMed]
- den Uil CA, Daemen J, Lenzen MJ, et al. Pulsatile iVAC 2L circulatory support in high-risk percutaneous coronary intervention. EuroIntervention 2017;12:1689-96. [Crossref] [PubMed]
- Maly J, Ivak P, Netuka I, Herman A, et al. Initial experience with the HeartMate percutaneous heart pump in circulatory failure. J Heart Lung Transplant 2017;36:1016-9. [Crossref] [PubMed]
- Anderson MB, Goldstein J, Milano C, et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant 2015;34:1549-60. [Crossref] [PubMed]
- John R, Long JW, Massey HT, et al. Outcomes of a multicenter trial of the Levitronix CentriMag ventricular assist system for short-term circulatory support. J Thorac Cardiovasc Surg 2011;141:932-9. [Crossref] [PubMed]
- Takeda K, Garan AR, Ando M, et al. Minimally invasive CentriMag ventricular assist device support integrated with extracorporeal membrane oxygenation in cardiogenic shock patients: a comparison with conventional CentriMag biventricular support configuration. Eur J Cardiothorac Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Kilic A, Shukrallah BN, Whitson BA. Initiation and management of adult veno-arterial extracorporeal life support. Ann Transl Med 2017;5:67. [Crossref] [PubMed]
- Bréchot N, Demondion P, Santi F, et al. Intra-aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial-extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care 2017.2048872617711169. [PubMed]
- Koeckert MS, Jorde UP, Naka Y, et al. Impella LP 2.5 for left ventricular unloading during venoarterial extracorporeal membrane oxygenation support. J Card Surg 2011;26:666-8. [Crossref] [PubMed]
- Naito N, Nishimura T, Iizuka K, et al. Novel Rotational Speed Modulation System Used With Venoarterial Extracorporeal Membrane Oxygenation. Ann Thorac Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017;63:60-7. [Crossref] [PubMed]
- Shah P, Pagani FD, Desai SS, et al. Outcomes of Patients Receiving Temporary Circulatory Support Before Durable Ventricular Assist Device. Ann Thorac Surg 2017;103:106-12. [Crossref] [PubMed]
- Rodriguez LE, Suarez EE, Loebe M, et al. Ventricular assist devices (VAD) therapy: new technology, new hope? Methodist Debakey Cardiovasc J 2013;9:32-7. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant 2012;31:117-26. [Crossref] [PubMed]
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant 2014;33:555-64. [Crossref] [PubMed]
- Maltais S, Kilic A, Nathan S, et al. PREVENtion of HeartMate II Pump Thrombosis Through Clinical Management: The PREVENT multi-center study. J Heart Lung Transplant 2017;36:1-12. [Crossref] [PubMed]
- Uriel N, Colombo PC, Cleveland JC, et al. Hemocompatibility-Related Outcomes in the MOMENTUM 3 Trial at 6 Months: A Randomized Controlled Study of a Fully Magnetically Levitated Pump in Advanced Heart Failure. Circulation 2017;135:2003-12. [Crossref] [PubMed]
- Krabatsch T, Netuka I, Schmitto JD, et al. Heartmate 3 fully magnetically levitated left ventricular assist device for the treatment of advanced heart failure -1 year results from the Ce mark trial. J Cardiothorac Surg 2017;12:23. [Crossref] [PubMed]
- Grinstein J, Rodgers D, Kalantari S, et al. HVAD Waveform Analysis as a Noninvasive Marker of Pulmonary Capillary Wedge Pressure: A First Step Toward the Development of a Smart Left Ventricular Assist Device Pump. ASAIO J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Cerier E, Lampert BC, Kilic A, et al. To ventricular assist devices or not: When is implantation of a ventricular assist device appropriate in advanced ambulatory heart failure? World J Cardiol 2016;8:695-702. [Crossref] [PubMed]
- Pagani FD, Aaronson KD, Kormos R, et al. The NHLBI REVIVE-IT study: Understanding its discontinuation in the context of current left ventricular assist device therapy. J Heart Lung Transplant 2016;35:1277-83. [Crossref] [PubMed]
- Estep JD, Starling RC, Horstmanshof DA, et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: Results From the ROADMAP Study. J Am Coll Cardiol 2015;66:1747-61. [Crossref] [PubMed]
- Starling RC, Estep JD, Horstmanshof DA, et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: The ROADMAP Study 2-Year Results. JACC Heart Fail 2017;5:518-27. [Crossref] [PubMed]
- Bottio T, Bejko J, Gallo M, et al. Less invasive implantation of HeartWare left ventricular assist device. Multimed Man Cardiothorac Surg 2014.14. [PubMed]
- Strueber M, Meyer AL, Feussner M, et al. A minimally invasive off-pump implantation technique for continuous-flow left ventricular assist devices: early experience. J Heart Lung Transplant 2014;33:851-6. [Crossref] [PubMed]
- Sileshi B, Haglund NA, Davis ME, et al. In-hospital outcomes of a minimally invasive off-pump left thoracotomy approach using a centrifugal continuous-flow left ventricular assist device. J Heart Lung Transplant 2015;34:107-12. [Crossref] [PubMed]
- Khalpey Z, Smith R, Echeverria A, et al. A novel minimally invasive off-pump biventricular assist device insertion technique. J Thorac Cardiovasc Surg 2016;151:e5-7. [Crossref] [PubMed]
- Saeed D, Sixt S, Albert A, et al. Minimally invasive off-pump implantation of HeartMate 3 left ventricular assist device. J Thorac Cardiovasc Surg 2016;152:1446-7. [Crossref] [PubMed]
- Potapov EV, Kukucka M, Falk V, et al. Off-pump implantation of the HeartMate 3 left ventricular assist device through a bilateral thoracotomy approach. J Thorac Cardiovasc Surg 2017;153:104-5. [Crossref] [PubMed]
- Jeevanandam V. Yes, minimally invasive left ventricular device implantation can be done-but should it? J Thorac Cardiovasc Surg 2016;152:1448-9. [Crossref] [PubMed]
- Kilic A, Atluri P. Off-pump ventricular assist device implantation: Easy as 1, 2, 3? J Thorac Cardiovasc Surg 2017;153:106-7. [Crossref] [PubMed]
- Kilic A. The future of left ventricular assist devices. J Thorac Dis 2015;7:2188-93. [PubMed]
- Rich JD, Gosev I, Patel CB, et al. The incidence, risk factors, and outcomes associated with late right-sided heart failure in patients supported with an axial-flow left ventricular assist device. J Heart Lung Transplant 2017;36:50-8. [Crossref] [PubMed]
- Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol 2010;105:1030-5. [Crossref] [PubMed]
- LaRue SJ, Raymer DS, Pierce BR, et al. Clinical outcomes associated with INTERMACS-defined right heart failure after left ventricular assist device implantation. J Heart Lung Transplant 2017;36:475-7. [Crossref] [PubMed]
- Kavarana MN, Pessin-Minsley MS, Urtecho J, et al. Right ventricular dysfunction and organ failure in left ventricular assist device recipients: a continuing problem. Ann Thorac Surg 2002;73:745-50. [Crossref] [PubMed]
- Matthews JC, Koelling TM, Pagani FD, et al. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol 2008;51:2163-72. [Crossref] [PubMed]
- Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316-24. [Crossref] [PubMed]
- Kalogeropoulos AP, Al-Anbari R, Pekarek A, et al. The Right Ventricular Function After Left Ventricular Assist Device (RVF-LVAD) study: rationale and preliminary results. Eur Heart J Cardiovasc Imaging 2016;17:429-37. [Crossref] [PubMed]
- Cooley DA, Liotta D, Hallman GL, et al. Orthotopic cardiac prosthesis for two-staged cardiac replacement. Am J Cardiol 1969;24:723-30. [Crossref] [PubMed]
- Samak M, Fatullayev J, Sabashnikov A, et al. Past and Present of Total Artificial Heart Therapy: A Success Story. Med Sci Monit Basic Res 2015;21:183-90. [Crossref] [PubMed]
- Copeland JG, Smith RG, Arabia FA, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med 2004;351:859-67. [Crossref] [PubMed]
- Copeland JG, Copeland H, Gustafson M, et al. Experience with more than 100 total artificial heart implants. J Thorac Cardiovasc Surg 2012;143:727-34. [Crossref] [PubMed]
- Slepian MJ, Alemu Y, Girdhar G, et al. The Syncardia(™) total artificial heart: in vivo, in vitro, and computational modeling studies. J Biomech 2013;46:266-75. [Crossref] [PubMed]
- Wells D, Villa CR, Simón Morales DL. The 50/50 cc Total Artificial Heart Trial: Extending the Benefits of the Total Artificial Heart to Underserved Populations. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2017;20:16-9. [Crossref] [PubMed]
- Jansen P, van Oeveren W, Capel A, et al. In vitro haemocompatibility of a novel bioprosthetic total artificial heart. Eur J Cardiothorac Surg 2012;41:e166-72. [Crossref] [PubMed]
- Carpentier A, Latrémouille C, Cholley B, et al. First clinical use of a bioprosthetic total artificial heart: report of two cases. Lancet 2015;386:1556-63. [Crossref] [PubMed]
- Smadja DM, Saubaméa B, Susen S, et al. Bioprosthetic Total Artificial Heart Induces a Profile of Acquired Hemocompatibility With Membranes Recellularization. J Am Coll Cardiol 2017;70:404-6. [Crossref] [PubMed]
- Horvath D, Byram N, Karimov JH, et al. Mechanism of Self-Regulation and In Vivo Performance of the Cleveland Clinic Continuous-Flow Total Artificial Heart. Artif Organs 2017;41:411-7. [Crossref] [PubMed]
- Kleinheyer M, Timms DL, Greatrex NA, et al. Pulsatile operation of the BiVACOR TAH - Motor design, control and hemodynamics. Conf Proc IEEE Eng Med Biol Soc 2014;2014:5659-62. [PubMed]
- Mehta SM, Pae WE, Rosenberg G, et al. The LionHeart LVD-2000: a completely implanted left ventricular assist device for chronic circulatory support. Ann Thorac Surg 2001;71:S156-61; discussion S183-4.
- Dowling RD, Gray LA, Etoch SW, et al. Initial experience with the AbioCor implantable replacement heart system. J Thorac Cardiovasc Surg 2004;127:131-41. [Crossref] [PubMed]
- Schmid Daners M, Kaufmann F, Amacher R, et al. Left Ventricular Assist Devices: Challenges Toward Sustaining Long-Term Patient Care. Ann Biomed Eng 2017;45:1836-51. [Crossref] [PubMed]
- Pauls JP, Nandakumar D, Horobin J, et al. The Effect of Compliant Inflow Cannulae on the Hemocompatibility of Rotary Blood Pump Circuits in an In Vitro Model. Artif Organs 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Gregory SD, Stevens MC, Pauls JP, et al. In Vivo Evaluation of Active and Passive Physiological Control Systems for Rotary Left and Right Ventricular Assist Devices. Artif Organs 2016;40:894-903. [Crossref] [PubMed]
- Noviani M, Jamiolkowski RM, Grenet JE, et al. Point-of-Care Rapid-Seeding Ventricular Assist Device with Blood-Derived Endothelial Cells to Create a Living Antithrombotic Coating. ASAIO J 2016;62:447-53. [Crossref] [PubMed]
- Robotti F, Franco D, Bänninger L, et al. The influence of surface micro-structure on endothelialization under supraphysiological wall shear stress. Biomaterials 2014;35:8479-86. [Crossref] [PubMed]
- Stefopoulos G, Robotti F, Falk V, et al. Endothelialization of Rationally Microtextured Surfaces with Minimal Cell Seeding Under Flow. Small 2016;12:4113-26. [Crossref] [PubMed]


