Study on the role of transient receptor potential C6 channels in esophageal squamous cell carcinoma radiosensitivity
Introduction
Esophageal cancer is a common malignant solid tumor, now ranking as the sixth leading cause of cancer-related death worldwide and the fourth cause death in China. It affects more than 450,000 people worldwide and the incidence is increasing rapidly (1-4). The overall 5-year survival ranges from 15% to 25%, and the best outcomes are associated with disease diagnosed in the early stages (5). There are two major types of esophageal cancers, squamous cell carcinoma and adenocarcinoma, and the esophageal squamous cell carcinoma (ESCC) has especially high morbidity and mortality in China (6). The high recurrence rate after surgery is a main problem. It has reported that adjuvant chemo-radiotherapy after surgery can raise the survival rate, and the radiotherapy dosage is one of the main reasons positively correlation to the treatment effectiveness (7). We usually choose lower radiotherapy dosage because of the main organs nearby the esophagus. So, the radiosensitivity is the important issue we have to research. Ca2+, as a ubiquitous second messenger, was reported to be a key role in control of gene expression, cell cycle progression, apoptosis, proliferation and differentiation in many cancers (8,9). Meanwhile, expression and activation of the channels in the transient receptor potential (TRP) superfamily were found in different cancers (10).
In our previous study (11), we have reported that expression of transient receptor potential C6 (TRPC6) in ESCC specimens was markedly enhanced than in normal tissue and that inhibition of TRPC6 suppressed ESCC cell growth, cell cycle arrest in G2/M phase in culture and in nude mice. We used cytosolic Ca2+ imaging to examine whether the TRPC6 channels in Eca109 cells were Ca2+ permeable, and we have found that TRPC6 channel was Ca2+ permeable and Ca2+ influx through TRPC6 channels was critical for G2/M phase transition in ESCC cells. So, we suggest that TRPC6 channel was an important role in the development of human esophageal cancer via regulation of cell cycle progression.
In this study, we have explained the mechanisms how TRPC6 regulated cell proliferation and cell cycle. Because of its function of G2/M cell cycle blocking, we have studied that TRPC6 channel inhibitor enhanced the effect of radiotherapy in vivo and in vitro, and TRPC6 may be a new target of gene therapy and radiosensitivity of ESCC.
Methods
Ethics statement
This study was approved by Ethics Committee of Zhongshan Hospital, Fudan University for research involving animals (No. 2016-155).
Cell growth assay
Cell growth was estimated by determination of the cell number and the colony formation. The cells were seeded at an initial density of 6×105 per 60 mm dish. After culture for 24 h the cells were treated with 5 µM SKF96365 from Calbiochem (Gibbstown, New Jersey, USA) for 0, 6, 12 and 24 h. The cells were then harvested at the indicated time and the numbers were counted using the COULTER (Beckman, Fullerton, California, USA).
Cell cycle analysis
Briefly, the cells harvested and fixed were incubated with 0.2 mg/mL RNase and 10 µg/mL propidium iodide (PI). They were then assayed on FACSCalibur (Becton-Dickinson, Franklin Lakes, New Jersey, USA) and cell cycle distributions were analysed by the CellQuest Pro software (Becton-Dickinson). All analyses were performed in triplicate.
Cell proliferation assay
The cells were seeded at an initial density of 6×105 per 60 mm dish, and then divided into four groups. After culture for 24 h, groups 1 and 2 were treated with 5 µM SKF96365 for 8 h. Then groups 1 and 3 were performed with radiotherapy with the dosage of 2 Gy per day continuously for 5 days. Group 4 received nothing. Briefly, experiments were grouped as follows:
- Group 1: SKF96365 + radiotherapy;
- Group 2: SKF96365 only;
- Group 3: radiotherapy only;
- Group 4: control.
All cells were harvested and analyzed the cell proliferation using WST-8 dye (Beyotime Inst Biotech, Haimen, Zhejiang, China) according to manufacturer’s instruction. Briefly, 5×103 cells/well was seeded in a 96-well flat-bottomed plate, grown at 37 °C for 24 h, and then dyed with 10 µL WST-8. All cells were incubated at 37 °C for 2 h and the absorbance was finally determined at 450 nm using a microplate reader.
Flow cytometry
The cells trypsinized and fixed were then resuspended in PBS containing 30 µg/mL PI and 100 µg/mL RNase. After incubation at 37 °C for 30 min, cells were subjected to cell cycle analysis in FACSCalibur (Becton-Dickinson) and data were analyzed by WinMDI software.
Animal
Forty-four-week-old male BALB/c nu/nu nude mice were housed in a specific pathogen-free environment. The Eca109 ESCC cells infected were harvested, washed, and suspended in PBS. Then, 7.5×105 cells in 200 µL were injected subcutaneously into the right flank regions. Mice were checked every 3 days for tumor appearance, and tumor sizes were determined by measuring 2 diameters with a caliper. Tumor volume (V) was estimated using the equation V = ab2/2, where “a” is the maximum length, and “b” is the maximum width. Ten mice were included in each group. Four days after tumor cells injected, 0.1 mL 5 µM SKF96365 was performed by intraperitoneal injection with the doze of 20 mg/Kg per day continuously for 7 days. Radiotherapy was performed after SKF96365 injected 2 days later with the dosage of 2 Gy per day continuously for 5 days. One group were performed only with radiotherapy as the same dosage, another group were performed only SKF96365 as the same. As control, the last group received only 0.1 mL normal saline by intraperitoneal injection. Briefly, animals were grouped as follows:
- Group 1: SKF96365 + radiotherapy;
- Group 2: SKF96265 only;
- Group 3: radiotherapy only;
- Group 4: control.
Animals were killed 42 days after tumor-cell implantation. All animal procedures were approved by local animal care and use committee.
Statistical analysis
All the experiments were performed at least 3 times, and all the values were represented as mean ± standard deviation (SD). The results were presented as the means with the 95% confidence intervals (CIs) and analyzed by the Student t-test. Differences were considered statistically significant at P<0.05.
Results
Blocking TRPC6 channels inhibited ESCC cell growth and arrested at G2/M phase
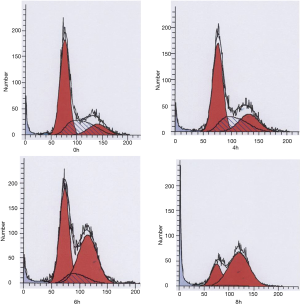
As shown in our previous study, SKF96365, a reagent known to inhibit TRPC channels (12), substantially increased the percentage of Eca109 cells in G2/M phase and reduce that in G0/G1 phase in a time-dependent manner. Most of the cells (85.26%), 24 h after the treatment, arrest in G2/M phase. In these experiments the cell population at subG0 phase was not changed, indicating that inhibiting TRPC6 did not induce apoptosis in these cells. These results suggested that Ca2+ TRP6 was critical for G2/M phase transition of the ESCC cells.
As shown in Figure 1, Eca109 ESCC cells performed with SKF96365 were mostly arrest at G2/M phase 8 h after SKF96365 treated. Taken together, these results suggested that inhibition of TRPC6 caused ESCC cells arrested in G2/M phase and subsequently suppressed their proliferation.
Blocking TRPC6 channels increased the sensitivity of radiotherapy of ESCC in vitro
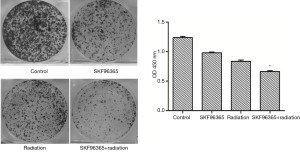
Based on the flow cytometry result, 8 h after SKF96365 treated, Eca109 cells were arrested mostly at G2/M phase without any apoptosis. As shown in Figure 2, Eca109 cells exposed to both radiotherapy and SKF96365 showed lowest ability of proliferation compared to those exposed to radiotherapy or to SKF96365 alone.
Blocking TRPC6 channels increased the sensitivity of radiotherapy of ESCC in nude mice
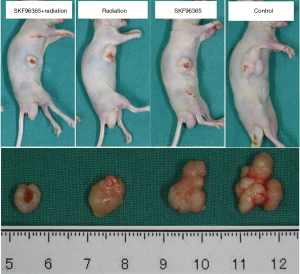
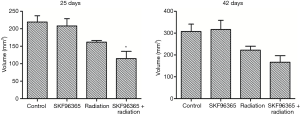
To provide direct evidence that cells arrested at G2/M phase are more sensitive to radiotherapy, we subcutaneously injected Eca109 cells into the flank of nude mice. A week after implantation, xenografted tumors could be seen. We then measured tumor sizes every 4 days within 1 month after implantation. When tumor sizes reached 1 cm3, the nude mice were received radiotherapy with the dosage of 2 Gy per day continuously for 5 days. Tumors formed by the cells transfected with SKF96365 grew much slower than those formed by the cells transfected with nothing. After 6 weeks, all nude mice were killed and the tumors were removed and weighed. At the macroscopic observation showed in Figure 3, the differences in tumor size and volume among the four groups were indicated. Statistical analysis showed the tumor mass in SKF96365 + radiotherapy, SKF96365, radiotherapy, or control group in Figure 4 (P<0.05, SKF96365 + radiotherapy vs. SKF96365 or radiotherapy). These results suggested that TRPC6 plays an important role in development of esophageal cancer, and SKF96365 may increase the sensitivity of radiotherapy. TRPC6 may become a new radiotherapy target in esophageal cancer.
Discussion
Esophageal cancer is the most rapidly increasing tumor type in the world. Globally, esophageal cancer is the sixth most common malignancy and fourth most fatal, with approximately 500,000 new diagnoses and more than 40, 000 deaths annually (1). In China, squamous cell carcinoma is the predominant histological type, which cost more than 90% cases. The high recurrence rate post-operation, the lower radio- and chemo-sensitivity are the main reason for the high mortality. It has reported that the radiotherapy dosage is one of the main reason positively correlation to the treatment effectiveness (13). We usually choose lower radiotherapy dosage because of the main organs nearby the esophagus. So, the radiosensitivity is the important issue we should face.
Ca2+ channel is the most compressive channel located on cell membrane (14). The abnormal expression of Ca2+ channel was found in more tumor tissues and cells (15-17). The high expression of Ca2+ channel in tumor may induce cellular proliferation, apoptosis dysfunction or cell migration, while lower expression or none was found in normal cells (18-21). TRPC6 is one of the most important Ca2+ channel protein, the abnormal change of TRPC6 may participate in tumorigenesis (22-24). This is the focus of the current research. In our previous study (11), the abnormal expression of TRPC6 was involved in ESCC growth, and its overexpression may induce tumor cell proliferation, and then break the balance between proliferation and apoptosis. If TRPC6 gene expression blockaded, human ESCC cells arrest mostly at G2/M phase, meanwhile, tumor cell proliferation decreased by 26%. It has been proved both in vivo and in vitro. Based on the theory, we used SKF96365, a reagent known to inhibit TRPC channels, to arrest human ESCC cell Eca109 to G2/M phase. To study the relationship between TRPC6 and radiotherapy sensitization, these arrested Eca109 cells were performed by radiotherapy. SKF96365 was injected before and after radiotherapy performed in order to synchronize cell cycle, so the curative effect may be raised. We have designed different concentration and different time of SKF96365 administrated in vitro, and we chose 5 µM and 8 h after SKF96365 treated. At this concentration and time, Eca109 cells were mostly arrest at G2/M phase, which was the best condition for radiotherapy (25).
It is important to understand the biological reaction of the cells to radiation to improve radiotherapy for malignant tumors. The reasons of failure for radiation treatment were that less dosage was given, or radiation was not properly fractionated (26). In addition, radioresistance and radiation-induced development of neoplasia contribute significantly to treatment failure (27,28). Cells exposed to radiation showed a temporary, reversible cell cycle arrest and then continued to proliferate at a slower rate. Checkpoints in the cell cycle regulate the progression or arrest of the cell cycle in the response to DNA damage and allow time for DNA repair. These occur either in late G1, which prevents entry to the S phase, or in late G2, which prevents entry to mitosis (29). Our study showed that, ESCC Eca109 cell treated with SKF96365 arrested mostly in the G2/M phase, which indicates entry into mitosis had been delayed. Thus, given that the G2/M checkpoint serves as a mandatory requirement for survival of ESCC, this delay in cell division, in combination with more efficient DNA damage repair, is necessary for maintenance of genome integrity in these cells. Both the extent and the length of this G2/M delay were reported be highly variable based on the cell line, radiation dose and the dose rate (30).
It is crucial to enhance tumor radiosensitivity for the purpose of both lowering the dose of ionizing radiation and achieving higher antitumor efficacy. We identified SKF96365 as a radiosensitizer to enhance ESCC cell response to radiotherapy in vitro and further investigated the mechanism mediating this effect. We treated ESCC Eca109 cell line with SKF96365, radiation and combined radio-SKF96365. Cell viability and cell cycle distribution were determined to ascertain the radiosensitization effect of SKF96365. Treatment with combined radio-SKF96365 led to decreased viability of Eca109 and had a profound radiosensitization effect. Pre-treatment with 5 µM SKF96365 increased radio-induced G2/M arrest in the cell cycle. This offers great potential for SKF96365 to be used in conjunction with radiotherapy for ESCC in order to increase the efficiency of the treatment.
Based on the study about TRPC6, we have found that inhibition of TRPC6 could arrest the ESCC cells at G2/M phase, which may influence cell proliferation. Meanwhile, the G2/M phase was the important checkpoint of radiosensitivity. We will use the tool of gene transfection for radiosensitivity research in vivo in the future, which may be a new therapeutic target for esophageal carcinoma radiation.
Acknowledgements
Funding: The work was supported by a grant from the Ph.D. Programs Foundation of Ministry of Education of China (No. 20130071120069).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by Ethics Committee of Zhongshan Hospital, Fudan University for research involving animals (No. 2016-155).
References
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49. [Crossref] [PubMed]
- Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 2005;97:142-6. [Crossref] [PubMed]
- Pennathur A, Farkas A, Krasinskas AM, et al. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg 2009;87:1048-54; discussion 1054-5. [Crossref] [PubMed]
- Luo ML, Shen XM, Zhang Y, et al. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res 2006;66:11690-9. [Crossref] [PubMed]
- Xu Y, Yu X, Chen Q, et al. Neoadjuvant versus adjuvant treatment: which one is better for resectable esophageal squamous cell carcinoma? World J Surg Oncol 2012;10:173. [Crossref] [PubMed]
- Clapham DE. Calcium signaling. Cell 2007;131:1047-58. [Crossref] [PubMed]
- Berridge MJ, Bootman MD, Lipp P. Calcium--a life and death signal. Nature 1998;395:645-8. [Crossref] [PubMed]
- Bödding M. TRP proteins and cancer. Cell Signal 2007;19:617-24. [Crossref] [PubMed]
- Shi Y, Ding X, He ZH, et al. Critical role of TRPC6 channels in G2 phase transition and the development of human oesophageal cancer. Gut 2009;58:1443-50. [Crossref] [PubMed]
- Franzius D, Hoth M, Penner R. Non-specific effects of calcium entry antagonists in mast cells. Pflugers Arch 1994;428:433-8. [Crossref] [PubMed]
- Kleinberg L, Gibson MK, Forastiere AA. Chemoradiotherapy for localized esophageal cancer: regimen selection and molecular mechanisms of radiosensitization. Nat Clin Pract Oncol 2007;4:282-94. [Crossref] [PubMed]
- Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer 2008;8:361-75. [Crossref] [PubMed]
- Guo L, Li ZS, Wang HL, et al. Carboxyamido-triazole inhibits proliferation of human breast cancer cells via G(2)/M cell cycle arrest and apoptosis. Eur J Pharmacol 2006;538:15-22. [Crossref] [PubMed]
- Lee JM, Davis FM, Roberts-Thomson SJ, et al. Ion channels and transporters in cancer. 4. Remodeling of Ca(2+) signaling in tumorigenesis: role of Ca(2+) transport. Am J Physiol Cell Physiol 2011;301:C969-76. [Crossref] [PubMed]
- Monteith GR, McAndrew D, Faddy HM, et al. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer 2007;7:519-30. [Crossref] [PubMed]
- Lu F, Chen H, Zhou C, et al. T-type Ca2+ channel expression in human esophageal carcinomas: a functional role in proliferation. Cell Calcium 2008;43:49-58. [Crossref] [PubMed]
- Wondergem R, Gong W, Monen SH, et al. Blocking swelling-activated chloride current inhibits mouse liver cell proliferation. J Physiol 2001;532:661-72. [Crossref] [PubMed]
- Panner A, Cribbs LL, Zainelli GM, et al. Variation of T-type calcium channel protein expression affects cell division of cultured tumor cells. Cell Calcium 2005;37:105-19. [Crossref] [PubMed]
- Strobl JS, Melkoumian Z, Peterson VA, et al. The cell death response to gamma-radiation in MCF-7 cells is enhanced by a neuroleptic drug, pimozide. Breast Cancer Res Treat 1998;51:83-95. [Crossref] [PubMed]
- Zeng B, Yuan C, Yang X, et al. TRPC channels and their splice variants are essential for promoting human ovarian cancer cell proliferation and tumorigenesis. Curr Cancer Drug Targets 2013;13:103-16. [Crossref] [PubMed]
- Ding X, He Z, Zhou K, et al. Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J Natl Cancer Inst 2010;102:1052-68. [Crossref] [PubMed]
- Prevarskaya N, Zhang L, Barritt G. TRP channels in cancer. Biochim Biophys Acta 2007;1772:937-46. [Crossref] [PubMed]
- Gogineni VR, Nalla AK, Gupta R, et al. Chk2-mediated G2/M cell cycle arrest maintains radiation resistance in malignant meningioma cells. Cancer Lett 2011;313:64-75. [Crossref] [PubMed]
- Kupersmith MJ, Warren FA, Newall J, et al. Irradiation of meningiomas of the intracranial anterior visual pathway. Ann Neurol 1987;21:131-7. [Crossref] [PubMed]
- Knizetova P, Darling JL, Bartek J. Vascular endothelial growth factor in astroglioma stem cell biology and response to therapy. J Cell Mol Med 2008;12:111-25. [Crossref] [PubMed]
- Umansky F, Shoshan Y, Rosenthal G, et al. Radiation-induced meningioma. Neurosurg Focus 2008;24:E7. [Crossref] [PubMed]
- Pietenpol JA, Stewart ZA. Cell cycle checkpoint signaling: cell cycle arrest versus apoptosis. Toxicology 2002;181-182:475-81. [Crossref] [PubMed]
- Tyagi AK, Singh RP, Agarwal C, et al. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest, and apoptosis. Clin Cancer Res 2002;8:3512-9. [PubMed]


