The prognosis of invasive adenocarcinoma presenting as ground-glass opacity on chest computed tomography after sublobar resection
Introduction
Lung adenocarcinoma is well characterized by its histologic heterogeneity (1). Recently, the use of chest computed tomography (CT) for lung cancer screening and early-stage lung cancer detection has increased (2), and with advances in technology, the detection of ground-glass opacity (GGO) has also increased remarkably in Asia. Several studies have shown that persistent GGO nodules on CT had a high risk of malignancy (3,4), and most of these nodules were adenocarcinomas.
According to the 2015 World Health Organization (WHO) classification of Tumours of the Lung (1), malignant nodules presenting as GGO are regarded as low-grade malignancies with two subtypes: adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA). These groups of tumours are correlated with a favourable prognosis after surgical resection.
Generally, GGO on a chest CT is considered to indicate a lepidic component, suggestive of AIS or MIA, which are grouped along a continuum as non-invasive or minimally invasive tumours. As a result, many surgeons choose sublobar resection (wedge resection or segmentectomy) for curative treatment of GGO nodules. The advantages of sublobar resection over lobectomy are clear. In patients with major comorbidities, sublobar resection may be technically easier and associated with fewer perioperative complications (5). Even if lobectomy is feasible, a lesser resection may help preserve lung capacity and function, facilitating any subsequent resection of a potential metachronous tumour (6).
However, GGO nodules do not always represent AIS or MIA. Indeed, in some cases, GGO nodules have been associated with invasive adenocarcinoma (7). Moreover, we have had experience with a pure GGO that was pathologically diagnosed as invasive adenocarcinoma after sublobar resection. Thus, we wanted to determine which histologic components (without lepidic component) are associated with GGO and clarify whether a complete lobectomy is necessary in this context.
This study primarily analyses GGO tumours histologically that were diagnosed as invasive adenocarcinoma and compares the prognosis of patients with sublobar resection and lobectomy in invasive adenocarcinoma presenting as GGO. We then investigate whether complete lobectomy is necessary after sublobar resection in invasive adenocarcinoma presenting as GGO nodules.
Methods
Patients
Between January 2007 and December 2014, 787 consecutive patients at Seoul St. Mary’s Hospital in Korea were diagnosed with stage I NSCLC and underwent surgical resection. Of this population, 540 patients were diagnosed with stage I adenocarcinoma. Patients who underwent incomplete resection were excluded. No patients included in the study received preoperative chemotherapy or radiotherapy.
Among 540 patients with stage I lung adenocarcinoma, 23 patients were excluded from the study because they had synchronous lung cancer or multiple GGO tumours. The study retrospectively enrolled 517 patients and assigned them to two groups according to their radiological features: GGO-predominant tumours or solid-predominant tumours. The clinicopathological characteristics and histologic characteristics were analysed in the GGO-predominant tumour group. In the GGO-predominant tumour group, a comparison was conducted between AIS/MIA and invasive adenocarcinoma. The histologic subtypes were analysed in invasive adenocarcinoma presenting as GGO-predominant tumour, and we examined which subtypes other than the lepidic component resembled GGO. We also compared the 5-year recurrence-free survival (RFS) of invasive adenocarcinoma presenting as GGO predominant in patients with sublobar resection and lobectomy. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital at the Catholic University of Korea (No. KC16RISI1031).
Radiologic evaluation and preoperative staging
Primary lesions were evaluated using thin-section CT images. All chest CT scans were obtained at full inspiration and were retrospectively examined for GGO nodules. The preoperative CT findings were reviewed by the authors with blind fashion from pathologic information. GGO is defined on a CT scan by increased hazy opacities in the lung parenchyma with preservation of the bronchial structures and vascular margins (8). The diameter of the tumour (T) was defined as the largest axial diameter of the lesion on the lung window setting. The diameter of consolidation (C) on the axial image on the lung window setting was also measured, where consolidation was defined as an area of increased opacification that completely obscured the underlying bronchial structures and vascular markings. GGO-predominant tumours were those with a C/T ratio ≤0.5, and solid-predominant tumours were those with a C/T ratio >0.5.
All patients underwent preoperative staging via chest CT and positron emission tomography (PET)/CT scan. Lymph node staging was achieved by contrast-enhanced chest CT and F-18-FDG-PET/CT scanning. Any nodes with short-axis diameters >10 mm on CT scan or with FDG uptakes greater than those of surrounding mediastinal structures were regarded as harboring metastases. However, high nodal FDG uptake was discounted in the presence of benign calcification or if unenhanced CT images showed high attenuation with distinct margins. FDG uptake by mediastinal lymph nodes that was largely symmetric and equivocal on PET/CT scans was interpreted as inflammatory reactivity (9,10). Invasive mediastinal lymph node staging (i.e., mediastinoscopy or endobronchial ultrasound-guided transbronchial needle aspiration) was done only in the patients with positive lymph nodes as above.
Follow-up evaluations
All patients were followed from the day of surgery. They were examined physically and by chest radiography every 3 months and by chest CT covering cervical to abdominal lesions every 6 months for the first 2 years. Thereafter, they were examined physically and by low-dose chest CT every 6 months up to 5 years. After 5 years, they were examined physically and by low-dose chest CT annually. We also checked the newly developed GGO nodule. The 2nd primary GGO is defined as newly developed persistent (more than 3 months) GGO nodule after surgery for primary lesion.
Surgical procedures
Sublobar resection included wedge resection and segmentectomy. Sublobar resection was performed in the high-risk subgroup of patients with decreased pulmonary function or a comorbid disease. In patients with a GGO nodule located near the visceral pleura, intentional sublobar resection was considered with the patient’s consent. The surgical procedures were determined depending on the surgeon’s preferences, and sublobar resection was more likely selected if an adequate resection margin could be obtained. When we considered sublobar resection, we chose wedge resection or segmentectomy according to the depth of nodule from the lung surface. Most cases obtained a sufficient resection margin in which the length was larger than the tumour diameter. Among the 25 patients who underwent sublobar resection of invasive adenocarcinoma presenting as a GGO-predominant nodule, the sublobar resection was performed intentionally in 22 patients (88%) who had normal pulmonary function, because of high-risk (pulmonary disease) comorbidity in 1 patient (4%), and due to previous pulmonary resection in 2 patients (8%).
Pathologic staging and histologic evaluation
All clinical specimens were examined by a pathology specialist, whose observations were recorded. To describe the histologic patterns of tumours, the occupancy ratio of each histologic component (lepidic, acinar, papillary, micropapillary, and solid) in the total tumour area was measured and recorded semiquantitatively in 5% increments according to the 2015 WHO classification of lung tumours (1). AIS and MIA were defined as small (≤3 cm), and solitary adenocarcinomas consisted of lepidic component without invasion (AIS) or with ≤5 mm invasion (MIA). Invasive adenocarcinomas were classified into one of several subtypes (acinar adenocarcinoma, papillary adenocarcinoma, micropapillary adenocarcinoma, lepidic adenocarcinoma, etc.).
Statistical analysis
Clinicopathological factors for each group were analysed with Student’s t-test or the Wilcoxon rank-sum test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables. Data for the interval between surgical resection and last follow-up visit were analysed via the Kaplan-Meier method using confirmed recurrences to calculate RFS. The survival of each group was compared with a log-rank test. A value of P<0.05 was considered statistically significant. Statistical analyses were performed using SPSS 19.0 software (IBM Corporation, Armonk, NY, USA).
Results
Among 517 patients with stage I lung adenocarcinoma, GGO-predominant tumours were found in 191 patients (36.9%), and solid-predominant tumours were found in 326 patients (63.1%). Of the 191 GGO-predominant tumour patients, 97 (50.8%) and 94 (49.2%) were assigned to AIS/MIA and invasive adenocarcinoma groups, respectively.
Comparison of AIS/MIA and invasive adenocarcinoma in GGO-predominant tumour
GGO-predominant tumour was divided into AIS/MIA and invasive adenocarcinoma, and we compared the clinicopathological characteristics between AIS/MIA and invasive adenocarcinoma (Table 1). The mean maximum standardized uptake value (SUVmax) of fluorodeoxyglucose on PET was higher in invasive adenocarcinoma than in AIS/MIA (1.6 vs. 0.8, P=0.003). In AIS/MIA, pure GGO accounted for 61.9%, while in invasive adenocarcinoma, pure GGO accounted for 21.3% (P<0.001). The mean C/T ratio of AIS/MIA and invasive adenocarcinoma was 0.09 and 0.27, respectively (P<0.001). The mean tumour size was larger in invasive adenocarcinoma than in AIS/MIA (1.8 vs. 1.2 cm, P<0.001).
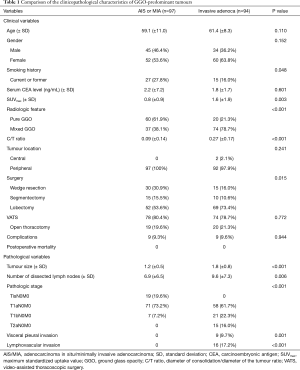
Full table
Analysis of subtypes and histologic components in GGO-predominant tumour
Histologic subtypes and the mean percentages of histologic component were analysed in AIS/MIA and invasive adenocarcinoma (Table 2). In invasive adenocarcinoma, the lepidic adenocarcinoma subtype accounted for 43.6%, acinar adenocarcinoma for 43.6%, papillary adenocarcinoma for 9.6%, and mucinous adenocarcinoma for 3.2%. The mean percentages of histologic components were calculated. In AIS/MIA, the mean percentage of the lepidic component was 91.8% and that of the acinar component was 7.3%. In invasive adenocarcinoma, the lepidic component was 47.4%, the acinar component was 42.1%, and the papillary component was 7.3%. As a result, GGO-predominant tumours mainly consisted of lepidic, acinar, and papillary components.

Full table
Analysis of histologic components in pure GGO tumours
We analysed the histologic subtypes and calculated the mean percentages of histologic components of pure GGO tumours (Table 3). Among them, AIS/MIA was 75.0% and invasive adenocarcinoma was 25.0%. Invasive adenocarcinoma consisted of acinar adenocarcinoma (11.2%), papillary adenocarcinoma (3.8%), and lepidic adenocarcinoma (10.0%). There were no subtypes such as micropapillary adenocarcinoma and solid adenocarcinoma. We analysed 20 patients who had invasive adenocarcinoma and pure GGO. The mean occupancy ratio of the histologic component was analysed. The mean percentage showed that the lepidic component (49.1%) and acinar component (43.7%) were the main components in invasive adenocarcinoma presenting as pure GGO. A papillary component (7.1%) was also present. However, micropapillary and solid components were not found. Therefore, the acinar and papillary components could also present as GGO.
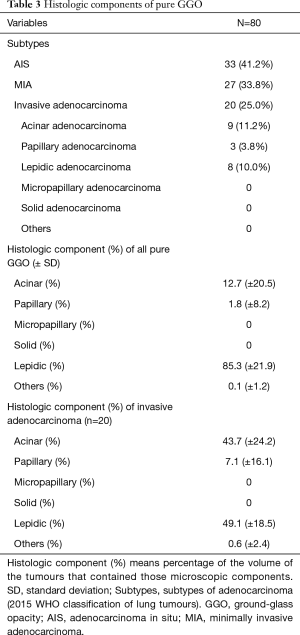
Full table
Comparisons of survival between sublobar resection and lobectomy
The median follow-up time for the sublobar resection group (n=25) was 1,071 days (range, 541–2,590 days), and that for the lobectomy group (n=69) was 1,277 days (range, 81–3,375 days).
We compared the clinicopathological characteristics of sublobar resection and lobectomy followed by a comparison of survival (Table 4). There were no significant differences between sublobar resection and lobectomy for most factors; the three significant differences were that SUVmax was lower in the sublobar resection group (0.8 vs. 1.8, P=0.033), the mean tumour size was smaller in sublobar resection than lobectomy (1.3 vs. 2.0 cm, P<0.001), and the number of dissected lymph nodes was lower in sublobar resection than lobectomy (3.7 vs. 11.8, P<0.001). The 5-year RFS rates of both sublobar resection and lobectomy were 100% in invasive adenocarcinoma presenting as a GGO tumour (Figure 1). The 5-year RFS rates of sublobar resection and lobectomy in AIS/MIA were also 100%. For reference, the 5-year RFS of solid-predominant tumours (sub-solid tumour) was 84.2%, and that of solid-predominant tumours (pure solid tumours) was 66.5% (Figure 1).
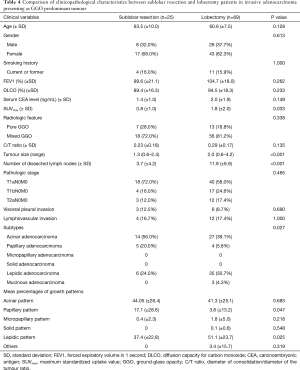
Full table
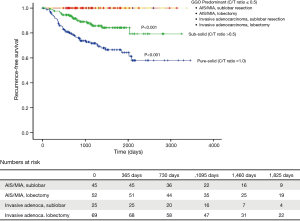
There was no recurrence of GGO-predominant tumours, regardless of the surgical approach (sublobar resection vs. lobectomy), but some newly developed GGO were found (Table 5) during the follow-up period. In a univariate analysis using the Cox-proportional hazard model, the occurrence of 2nd primary GGO was not associated with sublobar resection and histologic types (Table 6).

Full table
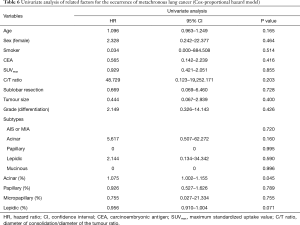
Full table
Discussion
In the present study, our aims were to determine what invasive components are associated with the presentation as GGO on chest CT and to evaluate the prognosis after sublobar resection of invasive adenocarcinoma presenting clinically as GGO. In this study, the acinar component and papillary components were related with GGO on chest CT. Our previous study showed that pure GGO tumours are not always composed of a lepidic component (7). Instead, acinar and papillary components may also present as GGO on chest CT. Other studies also support our results that pure GGOs consist of acinar or papillary components as well as lepidic component (11-13). Although the acinar and papillary components are invasive, the prognosis was not different with AIS or MIA if those tumours presented as GGO. Furthermore, the invasive adenocarcinoma presenting as GGO-predominant tumour showed a 100% 5-year RFS after sublobar resection in this study. Although we do not know whether all of those tumours will have 100% recurrent-free survival after surgery because of the small number of cases and short term follow up period, we can expect that the prognosis of those tumours is better than general invasive adenocarcinoma. Thus, additional complete lobectomy is not essential in this setting, despite the postoperative discovery of a predominant invasive component.
In this study, GGO-predominant tumours did not have any micropapillary adenocarcinoma or solid adenocarcinoma. According to previous studies on the subtypes of adenocarcinoma, micropapillary adenocarcinoma and solid adenocarcinoma have poorer prognosis than acinar adenocarcinoma and papillary adenocarcinoma (14-16). Some studies indicate that micropapillary adenocarcinoma and solid adenocarcinoma are high-grade malignant tumours and acinar adenocarcinoma and papillary adenocarcinoma are intermediate-grade malignant tumours (15-17). Therefore, we could expect a better prognosis for GGO-predominant tumours because these tumours do not contain high-grade malignant tumours. In addition, sublobar resection did not affect the prognosis of GGO-predominant tumour irrespective of histologic subtypes.
In addition, GGO-predominant tumours consisted of little micropapillary component and solid component in this study. Micropapillary and solid components were not found in pure GGO tumours. Micropapillary component is considered a poor prognostic component of adenocarcinoma (18). Although the subtype of the tumour is not a micropapillary adenocarcinoma, it was reported that tumours with a micropapillary component (>5%) have a poorer prognosis than tumours without a micropapillary component (19,20). It was also reported that the micropapillary component is associated with lymph node metastasis, especially nodal upstaging after surgical resection (10,21). As GGO-predominant tumours contain no or few micropapillary components, they have relatively less risk factors for lymph node metastasis and recurrence.
The C/T ratio is a well-established descriptor for GGO on chest CT, and it is easy to measure. In many studies, the C/T ratio was adopted as preoperative tumour characterization (22,23). We defined C/T ≤0.5 as indicative of a GGO-predominant tumour, and these tumours included only low-grade or intermediate grade malignant tumours, not high-grade malignant tumours. In this study, the mean C/T ratio of AIS or MIA was lower than that of invasive adenocarcinoma of GGO-predominant tumour. As the C/T ratio increased, the invasive component of the tumour increased. Therefore, the C/T ratio is a good indicator of tumour characteristics. Although 49% of the GGO-predominant tumours (C/T ≤0.5) were invasive adenocarcinomas, none of the recurrences occurred after sublobar resection, suggesting that the malignant potential was low even if the tumour was an invasive adenocarcinoma. Therefore, regardless of the histologic subtype of the tumour, the C/T ratio alone may be a good indicator of tumour malignant potential. In our previous study, we found little lymph node metastasis and no postoperative nodal upstaging in GGO-predominant tumours (24). In the present study, a 100% 5-year RFS after sublobar resection was reported in GGO-predominant tumours. Another study has reported no recurrence after segmentectomy in tumours with a C/T ratio ≤0.5 (23). Therefore, it is reasonable to determine the indication of sublobar resection using the C/T ratio. Additional complete lobectomy is unnecessary in GGO-predominant tumours whether the tumour is AIS/MIA or invasive adenocarcinoma.
In this study, we evaluated RFS instead of overall survival because in the case of stage I disease, more patients die from other causes than from the cancer during the follow-up period (19). Also, RFS is a more accurate measurement of survival analysis, since it reflects the biological behavior of the cancer rather than death due to unrelated factors.
Several study limitations are acknowledged. First, this was a retrospective review conducted at a single centre. Second, we obtained the data from a single institution, and the number of cases was relatively small. Specifically, the number of sublobar resection in invasive adenocarcinomas presenting as GGO was only 25 cases; nevertheless, it is clear that the 5-year RFS of sublobar resection in invasive adenocarcinomas presenting as GGO-predominant tumours was 100%. Therefore, our results can be considered meaningful. Third, the follow-up period was relatively short. Still, most recurrences of NSCLCs are known to occur postoperatively within a 2-year period (25), and early recurrence has been shown to mirror extended prognosis (26). Therefore, we think that our results are not meaningless. Finally, all data herein were clearly not homogeneous with regard to the comparison between sublobar resection and lobectomy of invasive adenocarcinoma presenting as a GGO-predominant tumour. Thus, the analytical outcomes are difficult to generalize. The present findings may be elaborated upon and refined through future studies with larger, less heterogeneous patient populations.
In conclusion, first, the GGO observed on CT is likely to be a lepidic component. However, as the invasive components such as acinar and papillary components can also be seen as GGO, not all GGO tumours are composed solely of lepidic components. Second, tissue can be diagnosed as invasive adenocarcinoma, even if it appears as GGO on CT. At this time, a good prognosis can be expected after sublobar resection, even with a diagnosis of invasive adenocarcinoma (acinar or papillary subtypes). Therefore, even if the final pathologic result is invasive adenocarcinoma after sublobar resection of GGO predominant tumours, routine follow-up rather than additional completion of lobectomy may be feasible.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital at the Catholic University of Korea (No. KC16RISI1031).
References
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Kim HY, Shim YM, Lee KS, et al. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology 2007;245:267-75. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Moon Y, Sung SW, Lee KY, et al. Pure ground-glass opacity on chest computed tomography: predictive factors for invasive adenocarcinoma. J Thorac Dis 2016;8:1561-70. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Lu P, Sun Y, Sun Y, et al. The role of (18)F-FDG PET/CT for evaluation of metastatic mediastinal lymph nodes in patients with lung squamous-cell carcinoma or adenocarcinoma. Lung Cancer 2014;85:53-8. [Crossref] [PubMed]
- Moon Y, Kim KS, Lee KY, et al. Clinicopathologic Factors Associated With Occult Lymph Node Metastasis in Patients With Clinically Diagnosed N0 Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1928-35. [Crossref] [PubMed]
- Wilshire CL, Louie BE, Manning KA, et al. Radiologic Evaluation of Small Lepidic Adenocarcinomas to Guide Decision Making in Surgical Resection. Ann Thorac Surg 2015;100:979-88. [Crossref] [PubMed]
- Sim HJ, Choi SH, Chae EJ, et al. Surgical management of pulmonary adenocarcinoma presenting as a pure ground-glass nodule. Eur J Cardiothorac Surg 2014;46:632-6; discussion 636. [Crossref] [PubMed]
- Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules >/= 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013;144:1291-9. [Crossref] [PubMed]
- Yoshiya T, Mimae T, Tsutani Y, et al. Prognostic Role of Subtype Classification in Small-Sized Pathologic N0 Invasive Lung Adenocarcinoma. Ann Thorac Surg 2016;102:1668-73. [Crossref] [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg 2014;98:453-8. [Crossref] [PubMed]
- Sasada S, Nakayama H, Miyata Y, et al. Comparison of Malignant Grade Between Pure and Partially Invasive Types of Early Lung Adenocarcinoma. Ann Thorac Surg 2015;99:956-60. [Crossref] [PubMed]
- Moon Y, Kim KS, Sung SW, et al. Correlation of histological components with tumor invasion in pulmonary adenocarcinoma. World J Surg Oncol 2014;12:388. [Crossref] [PubMed]
- Eguchi T, Kadota K, Park BJ, et al. The New IASLC-ATS-ERS Lung Adenocarcinoma Classification: What the Surgeon Should Know. Semin Thorac Cardiovasc Surg 2014;26:210-22. [Crossref] [PubMed]
- Zhang Y, Wang R, Cai D, et al. A comprehensive investigation of molecular features and prognosis of lung adenocarcinoma with micropapillary component. J Thorac Oncol 2014;9:1772-8. [Crossref] [PubMed]
- Moon Y, Lee KY, Kim KS, et al. Clinicopathologic correlates of postoperative N1 or N2 nodal upstaging in non-small cell lung cancer. J Thorac Dis 2016;8:79-85. [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Oncological Characteristics of Radiological Invasive Adenocarcinoma with Additional Ground-Glass Nodules on Initial Thin-Section Computed Tomography: Comparison with Solitary Invasive Adenocarcinoma. J Thorac Oncol 2016;11:729-36. [Crossref] [PubMed]
- Nishio W, Yoshimura M, Maniwa Y, et al. Re-Assessment of Intentional Extended Segmentectomy for Clinical T1aN0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:1702-10. [Crossref] [PubMed]
- Moon Y, Sung SW, Namkoong M, et al. The effectiveness of mediastinal lymph node evaluation in a patient with ground glass opacity tumor. J Thorac Dis 2016;8:2617-25. [Crossref] [PubMed]
- Tremblay L, Deslauriers J. What is the most practical, optimal, and cost effective method for performing follow-up after lung cancer surgery, and by whom should it be done? Thorac Surg Clin 2013;23:429-36. [Crossref] [PubMed]
- Kiankhooy A, Taylor MD, LaPar DJ, et al. Predictors of early recurrence for node-negative t1 to t2b non-small cell lung cancer. Ann Thorac Surg 2014;98:1175-83. [Crossref] [PubMed]


