Successful treatment with afatinib for pancreatic metastasis of lung adenocarcinoma: a case report
Case presentation
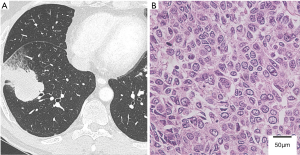
A 53-year-old asymptomatic man was referred to our hospital because of an abnormal shadow on the right lung on radiography. His Eastern Cooperative Oncology Group (ECOG) Performance Status was 0. Computed tomography (CT) showed a 4.5-cm mass in the right lower lobe (Figure 1A) and positron emission tomography-computed tomography (PET-CT) revealed accumulation of fluorine-18-deoxyglucose (FDG) in the mass. The patient underwent a video-assisted thoracoscopic right lower lobectomy and was diagnosed with acinar adenocarcinoma, pT2bN2M0 stage IIIA (Figure 1B). An epidermal growth factor receptor (EGFR) mutation (exon 19 deletion L747-A750insP) was detected in the primary tumor. The patient underwent adjuvant chemotherapy with cisplatin and gemcitabine (1 cycle every 3 weeks of cisplatin, 40 mg/m2 on days 1 and 8, and gemcitabine, 1,000 mg/m2 on days 1 and 8).
PET-CT performed 7 months after surgery revealed a hypermetabolic mass in the right hilum, indicating local recurrence of lung cancer. The patient was treated with concurrent chemoradiotherapy [radiotherapy, 60 Gy in 30 fractions; chemotherapy, weekly paclitaxel (45 mg/m2) and carboplatin (area under the curve =2)]. However, the patient complained of headaches during this treatment, and multiple brain metastases were detected on magnetic resonance imaging. Although chemoradiotherapy succeeded in controlling hilar recurrence, it was ineffective against the brain metastases. Thus, these lesions were treated with gamma knife radiosurgery followed by daily oral doses of 150 mg erlotinib. After 21 days of administration, the patient developed severe diarrhea and fever, and erlotinib was withdrawn. Although the patient was re-challenged with erlotinib at daily oral doses of 100 mg after these symptoms disappeared, 5 days after re-challenge, serum aspartate transaminase and alanine transaminase levels had increased to 583 and 1,383 U/L, respectively (grade 4 toxicity according to the Common Terminology Criteria for Adverse Events, version 4.0). Erlotinib was therefore discontinued and the patient started undergoing routine follow-up.
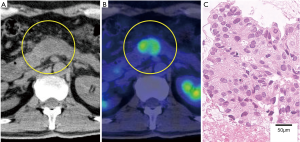
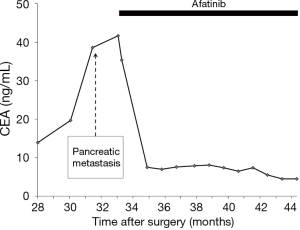
The patient complained of abdominal pain 17 months after discontinuance of erlotinib; his carcinoembryonic antigen (CEA) level had increased to 38.6 ng/mL. PET-CT revealed a 2.5-cm, hypermetabolic nodule in the pancreatic body but no other evidence of recurrence (Figure 2). Re-biopsy of the pancreatic mass was performed using endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA), and the nodule was pathologically diagnosed as a pancreatic metastasis of the previous lung adenocarcinoma. The EGFR status of the pancreatic metastasis was confirmed to be the same as that of the primary lung tumor [exon 19 deletion (del19) L747-A750insP with no evidence of T790M mutation]. Thus, afatinib was administered at daily oral doses of 40 mg. The patient’s abdominal pain immediately disappeared and his CEA level also decreased to approximately normal limits, though it transiently increased up to 41.7 ng/mL (Figure 3). Diarrhea was controllable using an antidiarrheal drug and hepatic toxicity remained below grade 1.
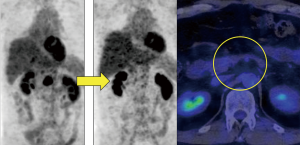
On a follow-up PET-CT scan obtained 10 months after treatment with afatinib, the metastatic nodule and accumulation of FDG in the pancreas had completely disappeared (Figure 4). Although some brain metastases previously treated using gamma knife radiosurgery recurred after 4 months of treatment with afatinib, they were successfully treated with additional gamma knife radiosurgery.
Discussion
Pancreatic metastasis of lung cancer is rare. Maeno et al. reported 26 cases of pancreatic metastasis among 850 lung cancer patients (3%), and a rate of 2.3% among patients with adenocarcinoma (1). Because of its rarity, an optimal treatment protocol for pancreatic metastatic lung cancer has not been established. To the best of our knowledge, ours is the first reported case in which pancreatic metastatic lung adenocarcinoma showed a complete response to EGFR-tyrosine kinase inhibitors (TKIs).
Patients in the clinical trials LUX-Lung 3 and 6 who received first-line afatinib had improved progression-free survival compared with those receiving cisplatin-based chemotherapy; moreover, those with the del19 EGFR mutation had improved overall survival (2). In the present case, the dramatic effect of afatinib on the pancreatic metastasis may have occurred due to a favorable EGFR mutation status.
EUS-FNA biopsy is an excellent, highly sensitive, and highly specific (86.8% and 95.5%, respectively) method of correctly diagnosing solid pancreatic masses (3). Re-biopsy of metastatic sites is essential because the EGFR mutation status is crucial for deciding the appropriate drug regimen in patients pretreated with TKIs (4).
It is important to consider possible adverse effects when deciding which TKIs to use. During initial treatment for the brain metastases, we had to discontinue erlotinib because of severe hepatotoxicity. When the pancreatic metastasis was diagnosed, afatinib was the preferred TKI because of the reported lower frequency of severe hepatotoxicity than treatment with gefitinib or erlotinib (5).
In the present case, afatinib did not completely control brain metastases. Nevertheless, Hoffknecht et al. have reported that 35% (11 of 31) of patients with non-small-cell lung carcinoma had a cerebral response to afatinib (6). The effective concentration of afatinib in cerebrospinal fluid (CSF) has not been determined; however, the concentration of afatinib in the CSF of our patient might not have reached the effective level. Moreover, the T790M EGFR mutation is known to be heterogeneous when organs metastases are present (7). These may be the reasons why afatinib was ineffective against the brain metastases, though we did not confirm the concentration of afatinib in the CSF of our patient or the EGFR-mutation status of the brain metastases.
However, afatinib, in combination with gamma knife radiosurgery, completely controlled the pancreatic and brain metastases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The study participant provided informed consent for the findings of this case to be published.
References
- Maeno T, Satoh H, Ishikawa H, et al. Patterns of pancreatic metastasis from lung cancer. Anticancer Res 1998;18:2881-4. [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Puli SR, Bechtold ML, Buxbaum JL, et al. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas 2013;42:20-6. [Crossref] [PubMed]
- Jekunen AP. Role of rebiopsy in relapsed non-small cell lung cancer for directing oncology treatments. J Oncol 2015;2015:809835. [PubMed]
- Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2015;88:74-9. [Crossref] [PubMed]
- Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol 2015;10:156-63. [Crossref] [PubMed]
- Suda K, Murakami I, Sakai K, et al. Heterogeneity in resistance mechanisms causes shorter duration of epidermal growth factor receptor kinase inhibitor treatment in lung cancer. Lung Cancer 2016;91:36-40. [Crossref] [PubMed]


