Could thymomectomy be a reasonable option for non-myasthenic thymoma patients?
Introduction
Thymoma is the most common tumour of the anterior mediastinum, with an incidence of 0.15 cases per 100,000 persons in the USA (1). Generally defined as an indolent neoplasm, it can occasionally manifest malignant behaviour by invading adjacent organs, seldom with pleural-pericardial or distant metastasis (2,3). Surgery is the mainstay of treatment for most thymomas, while irradiation and chemotherapy are commonly administered to non-resectable tumours or in adjuvant regimens (1,2,4). The possible association of thymoma with myasthenia gravis (MG) involves some peculiarities in the surgical treatment (5). Many studies recommend performing extended thymectomy as it is capable of treating both the tumour and myasthenia, even in non-myasthenic patients (6,7). In fact, the late onset of MG has been observed several years after the resection of thymoma, even without tumour recurrence (5,8). Moreover, in large series, several cases of thymomectomy with wide clear margin without either postoperative MG or tumour recurrence have been reported (9-11). Therein, a benefit from extended thymectomy for non-myasthenic thymoma patients has not been demonstrated (12-15). Specifically, the removal of the entire thymus gland is usually carried out to prevent the future occurrence of MG rather than for proved oncological aims (7,15). However, the potential role of extended thymectomy in preventing the development of MG in non-myasthenic thymoma patients has not been confirmed (5,8). The rationale for extended thymectomy in non-myasthenic patients is also weakened by the extremely rare finding of multicentric thymomas within the resected gland (16,17). A complete resection of thymoma, as well as Masaoka stage, World Health Organization (WHO) histological type and tumour size, is a strong prognostic factor for survival (18-20). Currently, only few studies have investigated the extension of the resection in the healthy thymus (12-15). Additionally, video-assisted thoracoscopic surgery has led to a frequent resection of small thymomas in non-myasthenic patients through tumour resection without an extended thymectomy (6,16,21).
The aim of this retrospective study was to compare extended thymectomy vs. thymomectomy in non-myasthenic thymoma patients for (I) oncological outcome, (II) multicentric thymoma occurrence and (III) postoperative MG development.
Methods
From March 1996 to September 2015, 160 consecutive patients were treated for thymoma at the Thoracic Surgery Units of Santa Maria della Misericordia Hospital, Perugia, and Santa Maria Hospital, Terni, Italy. The study was approved by the Institutional Review Board of Perugia and Terni University Hospitals (Code T1003).
Patients underwent a thorough neurological evaluation including: medical history, physical examination, anti-acetylcholine receptor antibodies (ARAb) assay and/or electromyogram (EMG) and/or edrophonium test to investigate MG.
According to the criteria of the Myasthenia Gravis Foundation of America, patients asymptomatic for MG but presenting with isolated high serum levels of ARAb (>0.4 mmol/L) or positive repetitive nerve stimulation studies were defined non-myasthenic (22).
Site, size and resectability of the neoplasms were established on the basis of CT-scan images for all patients and, when infiltration of the heart and great vessels was suspected, magnetic resonance imaging was performed. 18-FDG positron emission tomography was not routinely used, except for distinguishing thymic diseases with different biological behaviour, from thymic hyperplasia to thymic carcinoma. Mass biopsy was carried out for all non-resectable patients or whenever the judgement of the treating physician deemed it appropriate for atypical clinical behaviour.
Exclusion criteria included the following: thymic carcinoma (15 patients), surgical biopsy (12 patients), R2 resection (5 patients) and MG (36 patients). The clinical charts of the remaining 92/160 patients were analyzed. Patients were divided into two groups according to the extent of resection: extended thymectomy (70 patients) vs. R0-mediastinal thymomectomy (22 patients). Clinical data regarding symptomology, histology, oncological outcome and postoperative MG occurrence were compared between the two study groups.
Surgery
Extended thymectomy, defined as the removal of the thymoma and the thymic gland en bloc with the mediastinal fat bilaterally between the phrenic nerves and from the innominate vein to the diaphragm, was the procedure of choice for suspected/proven thymoma (6). The preferred approach was median sternotomy. Thymomectomy, that is the complete resection of the tumour leaving residual thymic tissue, was performed in the following cases: the presence of giant unilateral mass not removable through a median sternotomy, totally cervical thymoma, non-histologically proven small neoplasms easily resectable through video-thoracoscopy, thymoma removed during surgery for lung cancer and resectable mediastinal tumour with scattered pleural implants. When limited thymectomy was performed by thoracotomy/video-thoracoscopy, clamshell incision or cervicotomy, the tumour was resected intact with wide free margins by means of the “no touch” technique to avoid perforation of the capsule (2). For video-thoracoscopic surgery, the specimen was retrieved through a plastic bag to prevent pleural contamination (6). Whenever nodal involvement was apparent, interested lymph-nodes were removed, but systematic lymphadenectomy or sampling were not routinely performed. When infiltration of neighbouring organs was suspected, “en bloc” resection was performed on both the thymoma and any involved structures. Whenever feasible, pleural-pericardial implants and pulmonary metastasis were removed with free margins. Stage IV thymoma with complete removal of all neoplastic tissue was classified as having no microscopic or macroscopic residual tumour (R0).
All specimens were classified according to the WHO system: A, AB, B1, B2 or B3 thymoma and when tumours had more than one of these components, they were considered as the most aggressive type observed (23). Stage was assigned according to the Masaoka system: I, II, III IVA or IVB (2).
Adjuvant therapy
Over the 20-year study period, adjuvant therapy protocol was not entirely standardized. But generally speaking for our 92 patients, irradiation was delivered for invasive thymomas while chemotherapy was typically administered for stage IV thymomas.
Follow-up
Regular follow-up at an outpatient facility included physical examination, neurological evaluation, blood exams and CT scan every 6–9 months for the first 2 years, annually for the third, fourth and fifth postoperative years and afterwards every 24–36 months for another 10 years. Additional follow up data were obtained directly from patients themselves or their general practitioners. Recurrences were classified as local, whenever they occurred in the anterior mediastinum or tissues immediately contiguous to the resected thymoma; regional when they were intrathoracic (pleural or pericardial implants) not contiguous to resected thymoma and distant when they had haematogenous or lymphogenous spread (24). The treatment of choice for recurrence was iterative surgery, often followed by adjuvant therapy (19). Whenever surgery was not possible, due to the tumour site, patient age or comorbidities, patients underwent chemo-irradiation.
Statistical analysis
For all the variables, we performed a Pearson’s chi-squared test (χ2) to evaluate how likely it was that any observed difference between the sets arose by chance. For tests on the equality of means, we used unpaired two-samples Student’s t-test. A correlation study was performed by using the Pearson product-moment correlation coefficient.
Median follow-up time was calculated using the reverse Kaplan-Meier method. Overall survival, disease-free survival and freedom from recurrence curves were estimated by the Kaplan-Meier method. All estimates were achieved using STATA 14.2 (Stata Corp Ltd., College Station, TX, USA).
Results
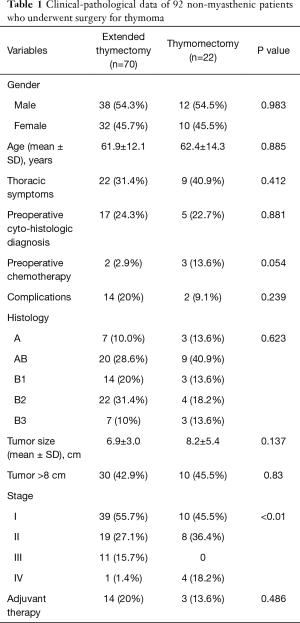
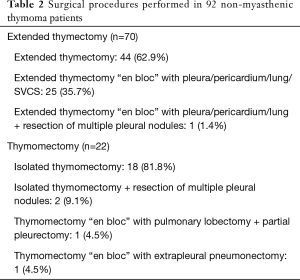
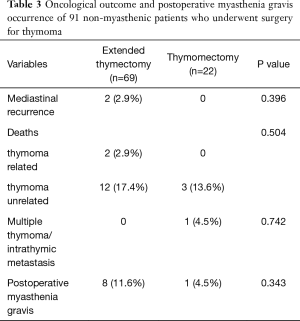
The clinical-pathological data of the 92 non-myasthenic thymoma patients, classified as either extended thymectomy (70 patients) and thymomectomy (22 patients) are shown in Table 1. Preoperative cytohistologic diagnosis by fine needle aspiration or incisional biopsy was performed in 22 patients, but in 4/22 (18.2%) patients the diagnosis of thymoma was not achieved. Surgical procedures performed on the two groups are shown in Table 2. The surgical approach for extended thymectomy was median sternotomy in 69/70 (98.6%) cases while only 1 (1.4%) case underwent combined sternothoracotomy. The latter approach was adopted in order to best manage adhesions/infiltration of the tumour to the phrenic nerve and the pulmonary hilum. The surgical approach for thymomectomy was thoracotomy in 14/22 (63.6%): 12/14 with large masses (median 12.5 cm; range 8–20 cm) and 2/14 with thymoma removed during lung cancer surgery. Video-thoracoscopy was performed for 6/22 (27.3%) tumours smaller than 5 cm. A totally cervical thymoma (4.5%) was resected intact through a cervical collar incision along with the upper thymus horn from which it originated (25). A clamshell incision was performed in 1 patient (4.5%) with hypovolemic shock for a bleeding 20 cm thymoma (26). There were no reported cases of postoperative mortality. For the extended thymectomy group, 7/70 (10%) experienced arrhythmia, 3/70 (4.3%) respiratory failure, 2/70 (2.9%) bleeding, 1/70 (1.4%) osteomyelitis and 1/70 (1.4%) lymphorrhea. Whereas for the thymomectomy group, we recorded 1/22 (4.5%) case of respiratory failure and 1/22 (4.5%) case of pericardial effusion. Regarding radical resection, all the 92 tumours were removed with macroscopically free surgical margins (R0). Nevertheless, there were 4/92 cases (4.3%) (1 in the first group and 3 in the second) where the mediastinal tumour was completely resected, but multiple pleural nodules could not be microscopically removed. The overall median follow-up period for the two groups was 77.4 months (range 1–255 months): 84 months (range 1–255 months) for extended thymectomy and 43 months (range 21–127 months) for thymomectomy. One patient who had undergone extended thymectomy, was lost during follow-up. Oncological outcomes and postoperative MG occurrences for both groups of patients are summarized in Table 3. There were no recorded tumour recurrences for the thymomectomy group (one radical completion thymectomy for metachronous thymoma), while 2/69 (2.9%) patients treated for B2 and B3 stage III thymomas experienced recurrences in the extended thymectomy group (P=0.396). Both of these patients died of thymoma progression. No thymoma related deaths were reported in the thymomectomy group (P=0.504). The aforementioned four cases in which multiple pleural nodules could not be removed, all had slow pleural disease progression without mediastinal recurrence. Figure 1 shows the overall survival, disease free survival and freedom from recurrence curves for both groups, respectively. The 5-year overall survival rates resulted being 97.1% (95% CI, 89.1–99.3%) for the extended thymectomy group and 90% (95% CI, 47.3–98.5%) for the thymomectomy group (P=0.590). The 5-year disease-free survival rates resulted being 92.4% (95% CI, 82.7–96.8%) and 75.1% (95% CI, 39–91.7%), respectively (P=0.175). Finally, the 5-year freedom from recurrence rates resulted being 95.1% (95% CI, 85.6–98.4%) and 85.9% (95% CI, 52.9–96.4%), respectively (P=0.203). There were no observed cases of either intrathymic metastases or multicentric thymomas for the extended thymectomy group. In one patient who had underwent thymomectomy, a single metachronous thymoma occurred in the remnant thymus, 37 months after surgery (P=0.742). Moreover, 8 cases (11.6%) of late onset of MG were registered for the extended thymectomy group (P=0.343). Only 1 patient from the thymomectomy group (4.5%) developed late postoperative MG, which was successfully treated with low-dose steroid therapy. Preoperative ARAb serum titer was assayed in 86/92 (93.5%) patients; in the remaining (6.5%) cases this exam was not performed as the diagnosis of thymoma was unexpected. High levels of ARAb serum titer were observed in 13/86 (15.1%) patients (range 0.4–16.5 nmol/L): 3/13 (23.1%) in the thymomectomy group, and 10/13 (76.9%) in the extended thymectomy group. Similar percentages of high preoperative ARAb serum titer were observed in the two groups: 3/19 (15.8%) in the thymomectomy group and 10/67 (14.9%) in the extended thymectomy group. Six (46.2%) of the aforementioned 13 patients with high preoperative ARAb serum titer developed postoperative MG whereas 7/13 (53.8%) did not. Among those nine patients in which postoperative MG occurred, 6/9 (66.7%) had high levels of ARAb serum titer preoperatively and 3/9 (33.3%) had normal ones. The interval between thymic surgery and the onset of MG ranged from 1 week to 37 months. A high preoperative ARAb serum titer assay was statistically related to postoperative MG occurrence (r=0.49, P<0.05).
Full table
Full table
Full table
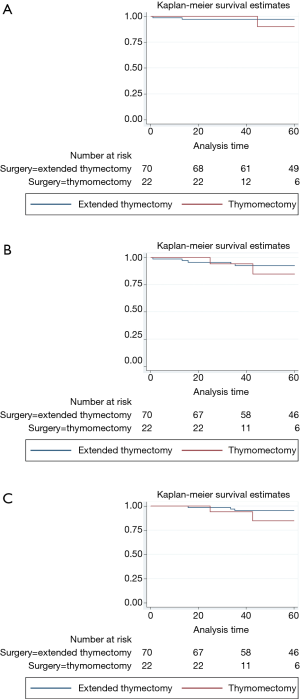
Even when only stage I–II thymoma patients (76 patients) were considered for statistical analysis, no statistically significant differences were observed in terms of thymoma recurrence (P=1.000), presence of multicentric thymoma (P=0.07) and MG occurrence (P=0.432) between the two groups. Similar results (P=1.000, 1.000, 0.698, respectively) were obtained when also giant masses were excluded (44 patients).
Discussion
Trans-sternal extended thymectomy is considered the standard procedure for the treatment of thymoma, irrespective of the presence of MG (6,7). Though the removal of the entire thymus gland and the mediastinal fat is strongly recommended for myasthenic thymoma patients, there is little evidence that extended thymectomy is necessary for non-myasthenic patients (12-15). In fact, current international recommendations have been based upon retrospective cohort studies and not prospective randomized trials (4,16,20). Furthermore, regarding the former studies, thymomectomy for non-myasthenic thymoma has been frequently reported (9-11). To date, few study results have been published in the English language on how the mode of resection can affect the prognosis of non-myasthenic thymoma patients (12-16).
The question whether thymomectomy could be a reasonable intervention for non-myasthenic thymoma patients could be answered through a comparative analysis with extended thymectomy, for what concerns: (I) oncological outcome, (II) multicentric thymoma occurrence and (III) postoperative MG development.
- It is widely known that the risk of recurrence is correlated to Masaoka stage, tumor size, WHO histological classification and completeness of resection (3,18-20,27). Few studies have investigated on how the extent of resection on the healthy thymus may affect the prognosis. To this regard, some authors have not reported any statistically significant differences between the two groups, concluding that limited thymectomy could be indicated for early stage thymoma only whenever microscopically free margins can be obtained (12,13,15,16,21,28,29). Likewise, Bae, when also including stages III and IV thymomas, reported a 9.7% recurrence rate in the limited thymectomy group and a 12.1% rate in the extended thymectomy group (14). Conversely, Murakawa and Wang, reported that extended thymectomy patients showed better prognosis compared to thymomectomy ones (30,31). Recently, two retrospective studies based on large series evaluated the role of the extent of the resection on the healthy thymus. Gu observed a significantly higher risk of tumour recurrence after thymomectomy, compared to total thymectomy, only for stage II tumours (14.5% vs. 2.9%; P=0,001). However, a significantly higher percentage of thymic carcinoma was reported for thymomectomy, compared to total thymectomy (17.1% vs. 6.4%; P=0,007) (32). Additionally, analyzing the ITMIG retrospective database, Burt identified total thymectomy as an independent variable associated with R0 resection. Nevertheless, his data showed comparable rates of R0 resection between minimally invasive and open surgery groups, despite a higher percentage of partial thymectomy in the first group (27% vs. 9%) (7).
- Multiple thymoma is rare, with a reported global incidence of 1–3.1% (17,33). The first three cases were published by Bernatz in 1961 (34). Fifty years later, Suzuki analyzed the existing 16 published cases, stating that only three of these were multicentric thymomas, while 13 were intra-thymic metastases (17). Onuki reported multiple macroscopic thymoma preoperatively detected at CT scan in two patients; these two patients later underwent extended thymectomy (15). Finally, Nakagawa, Bae, Odaka and Sakamaki did not report any multiple thymomas and all of the Authors concluded that if multicentric thymoma occurred after thymomectomy, it could be treated with completion thymectomy (12,14,21,28).
- Some authors have reported an incidence of postoperative MG between 1.5% and 28% for patients operated for thymoma without MG (5). Specifically, some authors have not reported any significantly different percentages, regarding the occurrence of postoperative MG, between the two groups (12,13,15), while Odaka and Sakamaki observed post-thymomectomy MG in two patients which were successfully treated with completion thymectomy 5 and 1 months after surgery. The latter were in agreement that the complete removal of the residual gland resulted been an effective treatment (21,28). As well, Sun and Kondo stated that the complete removal of thymic gland does not seem to prevent postoperative MG development, as in most series MG occurred after tumorectomy, thymo-thymectomy and extended thymectomy (5,8). The underlying biology of postoperative MG has not been identified, however high levels of ARAb in absence of tumour recurrence in these patients, suggest an extra-thymic process leading to the production of these antibodies. Results from the studies of Sun and Nakajima supported the hypothesis that preoperative high levels of ARAb in non-myasthenic thymoma patients may be a predictor of postoperative MG (5,35,36). The results of our study further support this hypothesis, as we observed that high preoperative ARAb serum titer assays were statistically correlated to postoperative MG occurrence (r=0.49, P<0.05).
In conclusion, in our series we did not observe any significant differences in the (I) oncological outcome, (II) multicentric thymoma occurrence and (III) postoperative MG development between the extended thymectomy and thymomectomy groups. Being so, thymomectomy might be a valid option for non-myasthenic thymoma patients especially with a patient selection where thymomectomy was performed not only for stage I–II small thymomas resectable through videothoracoscopy but also for giant unilateral masses, undiagnosed thoracic tumours later revealed as thymomas and neoplasms resected through a thoracotomy performed for lung cancer; as long as R0 mediastinal resection has been obtained.
There were several limitations to our study. First, it was a non-randomized retrospective single-institution study with a limited number of patients. Second, the median follow-up is longer for the group of extended thymectomy. Third, selection bias could have existed giving that the surgical strategy was decided by the surgeon. The results from ongoing analyses of prospective collaborative data collections, including that of ITMIG and ESTS Thymic Working Group, should provide greater insight on the role of thymomectomy for non-myasthenic thymoma patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Perugia and Terni University Hospitals (Code T1003). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Lattanzio R, La Sorda R, Facciolo F, et al. Thymic epithelial tumors express vascular endothelial growth factors and their receptors as potential targets of antiangiogenic therapy: A tissue micro array-based multicenter study. Lung Cancer 2014;85:191-6. [Crossref] [PubMed]
- Kondo K. Therapy for thymic epithelial tumors. Gen Thorac Cardiovasc Surg 2014;62:468-74. [Crossref] [PubMed]
- Wright CD, Wain JC, Wong DR, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization hystology, and size. J Thorac Cardiovasc Surg 2005;130:1413-21. [Crossref] [PubMed]
- Huang J, Ahmad U, Antonicelli A, et al. International Thymic Malignancy Interest Group International Database Committee and Contributors. Development of the international thymic malignancy interest group international database: an unprecedented resource for the study of a rare group of tumors. J Thorac Oncol 2014;9:1573-8. [Crossref] [PubMed]
- Sun XG, Wang YL, Liu YH, et al. Myasthenia gravis appearing after thymectomy. J Clin Neurosci 2011;18:57-60. [Crossref] [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Burt BM, Yao X, Shrager J, et al. Determinants of complete resection of thymoma by minimally invasive and open thymectomy: analysis of an International Registry. J Thorac Oncol 2017;12:129-36. [Crossref] [PubMed]
- Kondo K, Monden Y. Myasthenia gravis appearing after thymectomy for thymoma. Eur J Cardiothorac Surg 2005;28:22-5. [Crossref] [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [Crossref] [PubMed]
- Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995;60:908-13. [Crossref] [PubMed]
- Maggi G, Casadio C, Cavallo A, et al. Thymoma: results of 241 operated cases. Ann Thorac Surg 1991;51:152-6. [Crossref] [PubMed]
- Nakagawa K, Asamura H, Sakurai H, et al. Does the mode of surgical resection affect the prognosis/recurrence in patients with thymoma? J Surg Oncol 2014;109:179-83. [Crossref] [PubMed]
- Tseng YC, Hsieh CC, Huang HY, et al. Is thymectomy necessary in nonmyasthenic patients with early thymoma? J Thorac Oncol 2013;8:952-8. [Crossref] [PubMed]
- Bae MK, Lee SK, Kim HY, et al. Recurrence after thymoma resection according to the extent of the resection. J Cardiothorac Surg 2014;9:51. [Crossref] [PubMed]
- Onuki T, Ishikawa S, Iguchi K, et al. Limited thymectomy for stage I or II thymomas. Lung Cancer 2010;68:460-65. [Crossref] [PubMed]
- Narm KS, Lee CY, Do YW, et al. Korea Association for Research on the Thymus. Limited thymectomy as a potential alternative treatment option for early-stage thymoma: A multi-institutional propensity-matched study. Lung Cancer 2016;101:22-7. [Crossref] [PubMed]
- Suzuki H, Yoshida S, Hiroshima K, et al. Synchronous multiple thymoma: report of three cases. Surg Today 2010;40:456-9. [Crossref] [PubMed]
- Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg 2004;77:1860-9. [Crossref] [PubMed]
- Fiorelli A, D'Andrilli A, Vanni C, et al. Iterative Surgical Treatment for Repeated Recurrences After Complete Resection of Thymic Tumors. Ann Thorac Surg 2017;103:422-31. [Crossref] [PubMed]
- Ruffini E, Detterbeck F, Van Raemdonck D, et al. Tumors of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2014;46:361-8. [Crossref] [PubMed]
- Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. [Crossref] [PubMed]
- Jaretzki A 3rd, Aarli JA, Kaminski HJ, et al. Thymectomy for myasthenia gravis: evaluation requires controlled prospective studies. Ann Thorac Surg 2003;76:1-3. [Crossref] [PubMed]
- Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. [Crossref] [PubMed]
- Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. J Thorac Oncol 2011;6:S1691-7. [Crossref] [PubMed]
- Vannucci J, Tassi V, Monacelli M, et al. Totally cervical thymoma from the orthotopic thymus. Thorac Cardiovasc Surg 2012;60:175-6. [Crossref] [PubMed]
- Santoprete S, Ragusa M, Urbani M, et al. Shock induced by spontaneous rupture of a giant thymoma. Ann Thorac Surg 2007;83:1526-8. [Crossref] [PubMed]
- Vannucci J, Pecoriello R, Ragusa M, et al. Multiple pleuropericardial implants of thymoma after videothoracoscopic resection. Interact Cardiovasc Thorac Surg 2010;11:696-7. [Crossref] [PubMed]
- Sakamaki Y, Kido T, Yasukawa M. Alternative choices of total and partial thymectomy in video-assisted resection of noninvasive thymoma. Surg Endosc 2008;22:1272-7. [Crossref] [PubMed]
- Nakagawa K, Yokoi K, Nakajima J, et al. Is Thymomectomy alone appropriate for stage I (T1N0M0) thymoma? Results of a propensity-score analysis. Ann Thorac Surg 2016;101:520-6. [Crossref] [PubMed]
- Murakawa T, Nakajima J, Kohno T, et al. Results from surgical treatment for thymoma. 43 years of experience. Jpn J Thorac Cardiovasc Surg 2000;48:89-95. [Crossref] [PubMed]
- Wang LS, Huang MH, Lin TS, et al. Malignant thymoma. Cancer 1992;70:443-50. [Crossref] [PubMed]
- Gu Z, Fu J, Shen Y, et al. Thymectomy versus tumor resection for early-stage thymic malignancies: a Chinese Alliance for Research in Thymomas retrospective database analysis. J Thorac Dis 2016;8:680-6. [Crossref] [PubMed]
- Mori T, Nomori H, Ikeda K, et al. Three cases of multiple thymoma with a review of the literature. Jpn J Clin Oncol 2007;37:146-49. [Crossref] [PubMed]
- Bernatz PE, Harrison EG, Claget OT. Thymoma: a clinicopathologic study. J Thorac Cardiovasc Surg 1961;42:424-44. [PubMed]
- Nakajima J, Murakawa T, Fukami T, et al. Postthymectomy myasthenia gravis: relationship with thymoma and antiacetylcholine receptor antibody. Ann Thorac Surg 2008;86:941-5. [Crossref] [PubMed]
- Nakajima J, Okumura M, Yano M, et al. Myasthenia Gravis with thymic epithelial tumor: a retrospective analysis of a Japanese database. Eur J Cardiothorac Surg 2016;49:1510-5. [Crossref] [PubMed]


