Are beta blockers still necessary for all survivors of acute myocardial infarction?
In the previous decades, a huge number of clinical randomized controlled trials showed that beta blockers (BB) reduced incidence of all-cause death and adverse cardiac events in patients with acute myocardial infarction (AMI) (1-5). The Beta-blocker Heart Attack Trial (BHAT), which examined the efficacy of propranolol treatment initiated 5–21 days after AMI, showed that mortality was reduced by 25% during a mean follow-up period of 2 years (7% vs. 9.5%, respectively) (2,3). Similarly, the Norwegian trial, in which beneficial effect of timolol treatment from 6–27 days following AMI was examined, demonstrated nearly 40% reduction of mortality at 33 months (from 21.9% to 13.3%) (1). Furthermore, meta-analyses showed significant risk reduction via BB therapy for long-term mortality of post-AMI patents by 25% and 23% in the pre-reperfusion (6) and thrombolytic eras (7), respectively. Based on these findings, with respect to the of BB in the secondary prevention settings after ST-elevation myocardial infarction (STEMI), the ACCF/AHA guideline for the management of STEMI recommends BB should be continued during and after hospitalization for all patients with STEMI and with no contraindications to their use as a class I indication. Whereas, European Society of Cardiology (ESC) (8) as well as Japanese Circulation Society (JCS) (9) guidelines recommend BB use, in the absence of contraindications, in patients with reduced systolic left ventricular (LV) function [LV ejection fraction (EF) ≤40%] as a class I, and routine use of BB in all patients as a class IIa indication.
The favorable outcomes with BB treatment after AMI are likely attributable to protective effects for heart failure (HF) and sudden death caused by large infarct size, LV remodeling, residual ischemia, and LV arrhythmogenicity, and other factors (10-12). However, these benefits of BB could be reduced in the contemporary reperfusion era, since recent implementation of percutaneous coronary intervention (PCI) in acute managements of STEMI have greatly improved prognosis (13), possibly via limiting infarct size, residual ischemia, remodeling, and arrhythmia, for which BB have been prescribed to prevent for. In addition, we should notice that the mortality benefits of BB could have been further attenuated in the current era, since the increased implementation of evidence-based treatments other than PCI, such as cardiac rehabilitation, and administration of cardio-protective drugs including anti-platelets, renin-angiotensin system inhibitors, and statins, have likely reduced mortality (14), even without BB treatment. Thus, it is an emerging concern whether or how much BB treatment is still beneficial after AMI in the contemporary era.
In a recent issue of the Journal of American College of Cardiology, Dondo et al. addressed this issue by investigating the association between BB use and mortality in patients with AMI without HF or LV systolic dysfunction (LVSD) (15). A total of 179,810 survivors of hospitalization with AMI without HF or LVSD, between January 1, 2007, and June 30, 2013 (final follow-up: December 31, 2013) were derived from the Myocardial Ischaemia National Audit Project in the United Kingdom. Although unadjusted 1-year mortality was lower for patients who received β-blockers compared with those who did not, after weighting and adjustment of the clinical background with survival-time inverse probability weighting propensity scores and instrumental variable analyses, mortality of AMI patients did not differ between those with and without BB use, regardless of STEMI or non-STEMI. Although this study by Dondo et al. has a limitation that almost 95% of the study subjects had received BB, which likely affected the results as a medication bias even after the state-of the art statistical analysis, their findings were consistent with recent analyses (16-19) and likely true in the contemporary PCI era.
The REduction of Atherothrombosis for Continued Health (REACH) Registry, an observational study enrolling a total of 44,708 patients with either risk factors of coronary artery disease (CAD) only, known prior myocardial infarction (MI), or known CAD without MI, the use of BB was not associated with a lower risk of composite cardiovascular events in patients with prior MI as well as in the overall patients (16). In the Osaka Acute Coronary Insufficiency Study (OACIS) (17,20-23), we recently reported that mortality rates did not differ between patients with and without BB therapy (5.2% vs. 6.2%, P=0.786) during a median follow-up period of 1,430 days (adjusted hazard ratio 0.935, 95% confidence interval 0.711 to 1.230, P=0.534) in 5,628 consecutive survivors for STEMI admitted within 24 hours after onset and treated with primary PCI, which was confirmed in the matching cohort selected using propensity score (N=3,846, hazard ratio 0.834, 95% confidence interval 0.643 to 1.082, P=0.171) (17). Ozasa et al. also reported a similar finding from the J-Cypher Registry that BB was not associated with better 3-year clinical outcomes in 910 STEMI patients who underwent PCI within 24 hours from the onset (18). Furthermore, a recent meta-analysis comprising 16,645 patients without LVSD and received percutaneous PCI for AMI did not show prognostic superiority for the use of BB (19). These lines of evidence strongly suggest that benefits of BB may be limited in AMI survivors, at least, in those without LVSD or at lower mortality risk.
It should be noted, however, that, as compared with the current era, mortality risk after AMI was much higher in the pre-reperfusion and thrombolytic eras, in which previous large clinical trials established a firm evidence that BB treatment at discharge improved survival in post-MI patients (1-3,6,7). For example, the 2-year mortality rates in the Cooperative Cardiovascular Project, a retrospective analysis including over 200,000 post-MI patients, were 14.4% and 23.9% for those treated with BB and those without BB, respectively, even among low-risk individuals (4). In contrast, the overall mortality rates during a median follow-up period of 1,430 days in the OACIS study (17) were only 5.2% and 6.2% in the BB and non-BB groups, respectively, and particularly, mortality rates in non-BB group patients at low risk [Global Registry of Acute Coronary Events (GRACE) risk score (24) <121] was only 3.6%, which is significantly lower than those in the pre-reperfusion or thrombolytic eras (4,5). Importantly, however, even in the contemporary PCI era, the results from the OACIS study (17) also indicated benefits of BB therapy at discharge for high-risk patients: subgroup analyses among matched populations revealed that BB treatment was associated with a significantly decreased mortality for high-risk patients, who were defined as those with GRACE risk scores ≥121 or those administered diuretics, but not for lower risk patients (Figure 1), indicating that implementation of BB therapy for STEMI survivors may need to be assessed on the basis of individual mortality risk in the PCI era. Ozasa et al. also reported that BB treatment was associated with better survival (6.4% vs. 17.4%, P=0.04) and decreased incidence of major adverse cardiac events consisting of all-cause death, recurrent MI, and HF hospitalization in a subgroup of patients with LVEF of equal or less than 40% (18). In the OACIS study (17), however, survival classification and regression trees (CART) analysis (25) revealed no significant mortality risk reduction in patients with GRACE risk scores of ≥142 (Figure 2), while patients with GRACE risk scores between 121 and 141 were most benefitted by BB treatment with approximately 56% mortality risk reduction, indicating that the prescription of BB should be also considered with caution, particularly for individuals at extremely high risk.
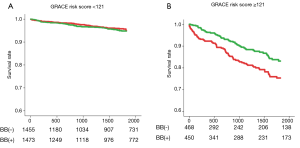
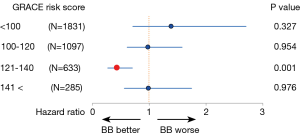
In conclusion, in the contemporary era, in which evidence-based strategies have been implemented, the clinical significance of BB treatment after AMI has been likely attenuated, as compared with that in the thrombolytic and pre-reperfusion era, particularly for the AMI survivors without LVSD, HF or other severe clinical conditions, while BB could be still effective in high-risk patients. However, it is a pity that there have been, to date, no randomized controlled trials that examined whether BB treatment reduces incidence of clinical outcomes for AMI in the PCI era. In this regard, reports from a randomized controlled trial, Carvedilol Post-intervention Long-term Administration in Large-scale Trial (CAPITAL-RCT) (ClinicalTrials.gov Identifier: NCT01155635), which aims to examine efficacy of carvedilol on 6-year clinical outcomes in patients with STEMI treated with PCI and preserved EF more than 40%, have been awaited. The results of CAPITAL-RCT would help us to address the clinical question whether BB treatment is still effective on long-term outcome in STEMI survivors without LVSD as well as to give us an opportunity to re-assess the current guidelines for secondary prevention after AMI.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med 1981;304:801-7. [Crossref] [PubMed]
- A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA 1982;247:1707-14. [Crossref] [PubMed]
- A randomized trial of propranolol in patients with acute myocardial infarction. II. Morbidity results. JAMA 1983;250:2814-9. [Crossref] [PubMed]
- Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med 1998;339:489-97. [Crossref] [PubMed]
- McMurray J, Køber L, Robertson M, et al. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J Am Coll Cardiol 2005;45:525-30. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal 2017. Available online: https://doi.org/ [Crossref]
- JCS Joint Working Group. Guidelines for secondary prevention of myocardial infarction (JCS 2011). Circ J 2013;77:231-48. [Crossref] [PubMed]
- Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985;27:335-71. [Crossref] [PubMed]
- Freemantle N, Cleland J, Young P, et al. beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999;318:1730-7. [Crossref] [PubMed]
- Frishman WH. Multifactorial actions of beta-adrenergic blocking drugs in ischemic heart disease: current concepts. Circulation 1983;67:I11-8. [PubMed]
- Cleland JG, Dargie HJ. Arrhythmias, catecholamines and electrolytes. Am J Cardiol 1988;62:55A-59A. [Crossref] [PubMed]
- Basu S, Senior R, Raval U, et al. Beneficial effects of intravenous and oral carvedilol treatment in acute myocardial infarction. A placebo-controlled, randomized trial. Circulation 1997;96:183-91. [Crossref] [PubMed]
- de Boer MJ, Hoorntje JC, Ottervanger JP, et al. Immediate coronary angioplasty versus intravenous streptokinase in acute myocardial infarction: left ventricular ejection fraction, hospital mortality and reinfarction. J Am Coll Cardiol 1994;23:1004-8. [Crossref] [PubMed]
- Tuppin P, Neumann A, Danchin N, et al. Evidence-based pharmacotherapy after myocardial infarction in France: adherence-associated factors and relationship with 30-month mortality and rehospitalization. Arch Cardiovasc Dis 2010;103:363-75. [Crossref] [PubMed]
- Dondo TB, Hall M, West RM, et al. β-blockers and mortality after acute myocardial infarction in patients without heart failure or ventricular dysfunction. J Am Coll Cardiol 2017;69:2710-20. [Crossref] [PubMed]
- Bangalore S, Steg G, Deedwania P, et al. beta-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA 2012;308:1340-9. [Crossref] [PubMed]
- Nakatani D, Sakata Y, Suna S, et al. Impact of beta blockade therapy on long-term mortality after ST-segment elevation acute myocardial infarction in the percutaneous coronary intervention era. Am J Cardiol 2013;111:457-64. [Crossref] [PubMed]
- Ozasa N, Kimura T, Morimoto T, et al. Lack of effect of oral beta-blocker therapy at discharge on long-term clinical outcomes of ST-segment elevation acute myocardial infarction after primary percutaneous coronary intervention. Am J Cardiol 2010;106:1225-33. [Crossref] [PubMed]
- Huang BT, Huang FY, Zuo ZL, et al. Meta-analysis of relation between oral beta-blocker therapy and outcomes in patients with acute myocardial infarction who underwent percutaneous coronary intervention. Am J Cardiol 2015;115:1529-38. [Crossref] [PubMed]
- Kurotobi T, Sato H, Kinjo K, et al. Reduced collateral circulation to the infarct-related artery in elderly patients with acute myocardial infarction. J Am Coll Cardiol 2004;44:28-34. [Crossref] [PubMed]
- Nakatani D, Sakata Y, Suna S, et al. Incidence, predictors, and subsequent mortality risk of recurrent myocardial infarction in patients following discharge for acute myocardial infarction. Circ J 2013;77:439-46. [Crossref] [PubMed]
- Hara M, Sakata Y, Nakatani D, et al. Comparison of 5-year survival after acute myocardial infarction using angiotensin-converting enzyme inhibitor versus angiotensin II receptor blocker. Am J Cardiol 2014;114:1-8. [Crossref] [PubMed]
- Masuda M, Nakatani D, Hikoso S, et al. Clinical impact of ventricular tachycardia and/or fibrillation during the acute phase of acute myocardial infarction on in-hospital and 5-year mortality rates in the percutaneous coronary intervention era. Circ J 2016;80:1539-47. [Crossref] [PubMed]
- Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA 2004;291:2727-33. [Crossref] [PubMed]
- Segal MR. Features of tree-structured survival analysis. Epidemiology 1997;8:344-6. [PubMed]


