Reoperative minimal access aortic valve replacement
Introduction
Recent frequent use of biological valves (1) and longer life expectancy has increased the number of reoperative aortic valve replacements (AVR). From 1997 to 2006, number of patients undergoing AVR who had previous coronary artery bypass grafting (CABG) has also increased by 60.4% (1).
Minimally invasive approaches for valve surgery were introduced in the late 1990s (2,3). Upper hemisternotomy is a typically used approach and has multiple clinical advantages such as decreased postoperative mechanical ventilation, bleeding and hospital stay (4,5). We adopted this approach for reoperative aortic valve replacement. Reoperative minimal access AVR (re-mini AVR) avoids the dissection of mediastinal tissues and protects previous grafts from iatrogenic trauma especially the left internal mammary artery (LIMA) to left anterior descending (LAD) artery (6). This approach is suitable for all re-AVR, but especially for high risk patients such as octogenarians (7).
Indication
Re-mini AVR is indicated for all isolated reoperative aortic valve replacements. Concomitant surgery in the ascending aorta can be performed.
Contraindications
Contraindications for re-mini AVR are the following:
- Need for CABG at the time of reoperation;
- Need for other valve (mitral, tricuspid) and/or root operation.
Technique
Preoperative work up
Three dimensional computed tomographies are performed in all patients undergoing re-mini AVR. This allows assessing the anatomic relationship between the sternum, the coronary graft(s), ventricle and the wires. Other routine tests such as coronary angiogram and echocardiogram are performed prior to the operation.
Pre-incision
Endotracheal intubation followed by pulmonary artery catheter insertion is performed by the anesthesiologist. A pacing port pulmonary artery catheter is used, due to the difficulty placing ventricular wire when re-mini AVR is performed. Transesophageal echocardiography (TEE) probe is inserted to allow monitoring of the valve insertion, left ventricular distention and the de-airing process. External defibrillator pads are attached to the patient prior to prepping and draping.
Cardiopulmonary bypass (CPB) access
Prior to sternotomy, CPB access is obtained. We use peripheral cannulation for almost all cases. For arterial access, right axillary artery or femoral artery is cannulated. Right axillary artery is the preferred approach. 6-10 mm polytetrafluoroethylene (PTFE) graft is sutured to the native artery as an interposition graft (Figure 1). For venous cannulation, percutaneous femoral venous approach is used. Using Seldinger technique, needle puncture followed by guidewire, dilator and venous cannula insertion is performed. Position of the cannula is confirmed using TEE.
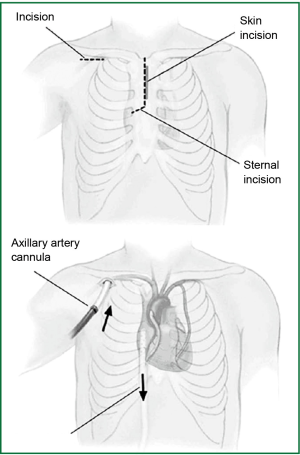
Reoperative sternotomy
The upper J-shaped hemisternotomy is performed to the 4th intercostal space using oscillating saw. CPB is established prior to dividing posterior table of the sternum. Once sternum is divided, sternal retracter is applied.
Mediastinal dissection
The key to this procedure is to limit the amount of mediastinal dissection. Dissection is performed around ascending aorta for application of aortic crossc-lamp and aortotomy. Only proximal aortocoronary graft is dissected and previous LIMA graft is left untouched.
CPB and cardioplegia strategy
If right atrial appendage and right superior pulmonary vein is easily accessible, retrograde cardioplegia catheter can be inserted from right atrial appendage and left ventricular vent can be inserted from right superior pulmonary vein. Antegrade cardioplegia and direct cardioplegia application to the coronary ostium is used when retrograde is not accessible. Direct left ventricular vent through aortotomy is used when pulmonary vein is not accessible.
On CPB, patient is cooled to 28-34 °C following aortic cross-clamp. Cardiac arrest is obtained through antegrade cardioplegia (and retrograde if available) with 20-30 min redosing through retrograde cardioplegia and/or coronary ostial direct cardioplegia. Left ventricular distention is monitored using TEE. AVR is performed using standard techniques (Figure 2).
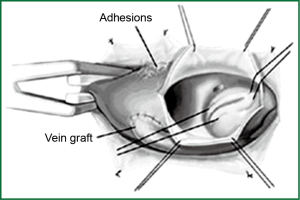
Patent LIMA graft
When a patent LIMA to LAD graft is present, constant blood is supplied to the myocardium through the subclavian artery which can cause ventricular fibrillation while blood return through coronary ostium obscures the view during annular suture placement. The goal of cooling is to achieve 25-30 °C with the patent LIMA. When cardiac activity is observed due to patent LIMA graft, additional systemic potassium (40 mEq) is given through the pump to a goal of 6.0-7.0 mEq. Ultrafiltration is used to lower the potassium level at the end of the case if this technique was used. When the field is obscured by blood return from the left coronary ostium, pump flow is temporarily lowered for a few seconds to place the annular sutures. Details of the steps and safety in patients with patent LIMA are discussed in previous publications (9).
Aortic crossclamp removal and closure
Defibrillation after unclamping is performed using external pads. Deairing is monitored by TEE and carbon dioxide is used in the field to limit the amount of air. A ventricular pacing wire is placed in right ventricular muscle if it is impossible to place a transvenous pacing wire due to technical or anatomical reasons. Chest tube is placed in the right pleural space and CPB is weaned and patient decannulated. Chest is closed in standard fashion.
Outcomes
Tam and associates reported the first successful case report of re-mini AVR using upper hemisternotomy in 1997 (10). Since then several case series as well as retrospective studies comparing re-mini AVR and conventional full sternotomy reoperative AVR have been published.
Case series of re-mini AVR
The largest case series to date confirming the safety of re-mini AVR is the series from Tabata and associates reporting outcomes of 146 patients who underwent re-mini AVR at BWH (8). The operative mortality was 4.1%, conversion to full sternotomy was 2.7%, reoperation was 0.7%, packed red blood cell (PRBC) requirement was 78.8% and median hospital stay was 8 days. Five year actuarial survival was 85%. There was no LIMA injury using this technique. They concluded that this approach is safe and feasible. Some small case series have reported no death (11,12), but most reported operative mortality of 3-6% (8,13-16) with minimal rates of complications.
Studies comparing re-min AVR and conventional full sternotomy reoperative AVR
The next question will be “what is the benefit of re-mini AVR compared to conventional full sternotomy reoperative AVR?”. Byrne and associates reported the first study comparing re-mini AVR and full re-AVR (6). They compared 20 patients who underwent re-mini AVR using upper hemisternotomy with 19 patients who underwent full sternotomy. There was no death in both groups and the trend toward less blood loss and transfusion requirement compared to full sternotomy.
Mihaljevic and associates compared 63 re-mini AVR to 134 conventional reoperative AVR in their series of 1,042 patients undergoing AVR at BWH (15). In hospital mortality (re-mini vs. full: 1.4% vs. 5%, P=0.33), reoperation for bleeding (0% vs. 2%, P=0.55) and length of stay (7 vs. 7, P=0.19) were all similar between two groups, although excellent results were obtained from re-mini AVR.
Sharony and associates compared 61 re-mini AVR with 160 full sternotomy AVR (16). Re-mini AVR was associated with lower in-hospital mortality (5.6% vs. 11.3%, P=0.04), fresh frozen plasma (FFP) requirement, deep sternal wound infection and shorter length of stay (7 vs. 8 days, P=0.009).
Pineda and associates compared 28 re-mini AVR with 40 patients who underwent full sternotomy reoperative AVR (17). Re-mini AVR was associated with lower composite postoperative complications, less prolonged ventilation, PRBC transfusion requirement. It also showed trend towards less in-hospital mortality (0% vs. 10%, P=0.09), shorter hospital stay and reoperation for bleeding.
To summarize, the published benefits of re-mini AVR over full re-AVR are lower operative mortality (16), less PRBC transfusion (6,17), less FFP transfusion (17), less blood loss and reoperation for bleeding (6,17), less prolonged ventilation (16) and shorter length of stay in the hospital (16,17).
Re-mini AVR for the elderly
Recently transcatheter aortic valve replacement (TAVR) has gained spotlight for patients with high risk. TAVR has established outcomes in patients with inoperable risks and has equal outcomes to surgery in high risk patients (18,19).
Kaneko and associates compared 51 octogenarians who underwent re-mini AVR to 54 octogenarians who underwent conventional re-AVR (7). Although operative mortality was similar between two groups (3.9% vs. 9.2%, P=0.438), Kaplan-Meier analysis showed survival benefit at both 1 year and 5 years (38%±17.6% vs. 65%±15.7%, P=0.028) favoring re-mini AVR. Predictors of mortality were heparin induced thrombocytopenia, reoperation for bleeding, increase in age, full sternotomy and infectious complication. They concluded that re-mini AVR may have advantage in these patients by limiting dissection and trauma in a patient group that has low tolerance to morbidity. Re-mini AVR may have a role even in high risk patients who have risks undergoing TAVR such as annular calcification extending to aorto-mitral curtain or low coronary ostium.
Controversies and concerns
Many surgeons throughout the world consider this approach as offering only cosmetic advantages. It is also true that this approach is performed only in limited centers. Promising outcomes of re-mini AVR have been reported throughout the world. However, this approach has not gained universal recognition as a standard approach. There has been no randomized control study to prove its benefit(s) over conventional full sternotomy approach.
This approach requires careful attention to myocardial protection especially in case of patent LIMA and coordination with perfusionist when systemic hyperkalemia and brief stopping of CPB is used. Not only good judgement from surgeons but also good teamwork by surgeon, anesthesiologist and perfusionist is required to successfully perform this minimally invasive procedure.
Conclusions
Re-mini AVR is a safe and feasible procedure. It limits the amount of mediastinal dissection hence less trauma to the patients. It also avoids dissection of LIMA graft and prevents injury to this critical bypass vessel. Benefits of this procedure over conventional full re-AVR include longer survival, less mortality, less blood loss and transfusion, shorter ventilation, less reoperation bleeding and shorter hospital stay, although no randomized control study has been performed to confirm these. This procedure may be particularly useful in high risk patients such as elderly to reduce morbidity and possibly increase their survival.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Brown JM, O’Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [PubMed]
- Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7. [PubMed]
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421-6; discussion 427-8. [PubMed]
- Liu J, Sidiropoulos A, Konertz W. Minimally invasive aortic valve replacement (AVR) compared to standard AVR. Eur J Cardiothorac Surg 1999;16 Suppl 2:S80-3. [PubMed]
- Mächler HE, Bergmann P, Anelli-Monti M, et al. Minimally invasive versus conventional aortic valve operations: a prospective study in 120 patients. Ann Thorac Surg 1999;67:1001-5. [PubMed]
- Byrne JG, Aranki SF, Couper GS, et al. Reoperative aortic valve replacement: partial upper hemisternotomy versus conventional full sternotomy. J Thorac Cardiovasc Surg 1999;118:991-7. [PubMed]
- Kaneko T, Loberman D, Gosev I, et al. Reoperative aortic valve replacement in the octogenarians-minimally invasive technique in the era of transcatheter valve replacement. J Thorac Cardiovasc Surg 2013. [Epub ahead of print]. [PubMed]
- Tabata M, Khalpey Z, Shekar PS, et al. Reoperative minimal access aortic valve surgery: minimal mediastinal dissection and minimal injury risk. J Thorac Cardiovasc Surg 2008;136:1564-8. [PubMed]
- Kaneko T, Nauta F, Borstlap W, et al. The “no-dissection” technique is safe for reoperative aortic valve replacement with a patent left internal thoracic artery graft. J Thorac Cardiovasc Surg 2012;144:1036-40. [PubMed]
- Tam RK, Garlick RB, Almeida AA. Minimally invasive redo aortic valve replacement. J Thorac Cardiovasc Surg 1997;114:682-3. [PubMed]
- Gaeta R, Lentini S, Raffa G, et al. Aortic valve replacement by ministernotomy in redo patients with previous left internal mammary artery patent grafts. Ann Thorac Cardiovasc Surg 2010;16:181-6. [PubMed]
- Svensson LG, Nadolny EM, Kimmel WA. Minimal access aortic surgery including re-operations. Eur J Cardiothorac Surg 2001;19:30-3. [PubMed]
- Totaro P, Carlini S, Pozzi M, et al. Minimally invasive approach for complex cardiac surgery procedures. Ann Thorac Surg 2009;88:462-6; discussion 467. [PubMed]
- Byrne JG, Karavas AN, Adams DH, et al. Partial upper re-sternotomy for aortic valve replacement or re-replacement after previous cardiac surgery. Eur J Cardiothorac Surg 2000;18:282-6. [PubMed]
- Mihaljevic T, Cohn LH, Unic D, et al. One thousand minimally invasive valve operations: early and late results. Ann Surg 2004;240:529-34; discussion 534. [PubMed]
- Sharony R, Grossi EA, Saunders PC, et al. Minimally invasive reoperative isolated valve surgery: early and mid-term results. J Card Surg 2006;21:240-4. [PubMed]
- Pineda AM, Santana O, Reyna J, et al. Outcomes of aortic valve replacement via right mini-thoracotomy versus median sternotomy after prior cardiac surgery. J Heart Dis 2011;8:54.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [PubMed]


