The role of bacterial culture by bronchoscopy in patients with lung cancer: a prospective study
Introduction
The interaction of microbes with host cells is known to have a major impact on the health of the host (1). Overall, there is an increasing interest in understanding the role of the tissue microbiome in carcinogenesis (2,3), with the understanding that certain pathogens may even cause chemotherapy resistance (4). Moreover, a recent study by Lee et al. using 16S rRNA-based next-generation sequencing (NGS) showed that differences exist in the bacterial communities of patients with lung cancer and those with benign mass-like lesions (5). They even suggested that the genera Veillonella and Megasphaera showed the potential to serve as biomarkers to predict lung cancer.
Although routine culture of bronchial aspirates may incur as an unnecessary expense and lead to over-diagnosis and overtreatment of patients with nonpathogenic bacteria (6,7), patients with lung cancer undergoing surgical and medical treatment are at increased risk for pulmonary complications (8-13). Targeted antibiotic therapy, based on bacterial culture of bronchial specimens, has been shown to reduce the risk of post-operative pneumonia (POP) in patients undergoing lung resection (14).
Many centers routinely examine bronchoscopy samples for bacteria even when infection is not strongly suspected. However, the importance of routinely examining these samples for bacteria in populations with lung cancer has rarely been defined. Since NGS is not yet routinely available in most centers, we wished to prospectively determine the types of potentially pathogenic microorganisms (PPM) among patients evaluated by bronchoscopy for suspected lung cancer in our medical center. Our aim was to test whether the ‘traditional’ microbiology methods would be sufficient to distinguish between lung cancer and those with benign mass-like lesions. In addition, we wished to evaluate the rate of potentially undiagnosed patients that would benefit from antibiotic treatment to prevent POP.
Methods
Study design
The prospective study group consisted of 155 consecutive patients with suspected malignancy that underwent bronchoscopic examination at our Pulmonary Institute from June 2011 through June 2012. Data on demographic characteristics, presenting symptoms, autoimmune status, chest X-ray and high resolution computed tomography (HRCT) of the chest were collected and the results of sample cultures were reviewed. Among the 155 patients, 93 proved to have a final diagnosis of lung cancer.
Bronchoscopy
All bronchoscopies were performed via trans-nasal insertion under conscious sedation by a board certified pulmonologist using an Olympus Video 240 P/140 P bronchoscope. Premedication consisted of 2.5–10 mg midazolam and 0.5–1 mg fentanil. A local anesthetic (3 mL of 1% lidocaine aerosol) was applied to the pharynx 15 minutes before the procedure. Bronchial washings were obtained by injecting 20 mL of isotonic saline through the bronchoscope into the affected segment, followed by immediate suction. The procedure was repeated 2 to 3 times, until 30 to 60 mL of material was collected from each patient. The washed material was cultured and subjected to staining for bacteria. The cultures were incubated for 1 week and examined 3 times during the week. Bronchial or transbronchial biopsy was performed when indicated by radiologic or bronchoscopic findings.
Clinical infection assessment
Clinical infection was defined by the presence of fever, cough, productive sputum or pleuritic chest pain according to American Thoracic Society (ATS) guidelines (15) and assessed up to 24 hours before the procedure. The final diagnosis was determined by the biopsy results and correlation with clinical, microbiological and radiological data.
Microbiological processing
A culture-positive specimen was defined as the growth of bacteria. Thresholds for quantitative cultures to define colonization were ≥103 colony-forming units (CFU)·mL−1. All microorganisms isolated were identified by standard laboratory methods (16). Then, the isolated bacterial agents were classified as PPMs or non-PPMs, in consistency with previous reports (8,10,11). The PPM group included microorganisms usually implicated in respiratory infections (e.g., Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Pseudomonas aeruginosa, Klebsiella pneumoniae, etc.). Non-PPMs included microorganisms not usually involved in respiratory infections in non-immunosuppressed hosts (e.g., Streptococcus viridans, Corynebacterium spp., Candida spp., coagulase-negative Staphylococcus etc.) (17).
Statistical analysis
The data was presented as percentage for non-metrics parameters or mean ± standard deviation (SD) for continuous variables. The mean age was compared by using student’s t-test between parameters. Nominal variables were analyzed by Chi-Square or Fisher’s exact test, where P<0.05 was considered statistically significant. All statistical analyses were computed by the SPSS 23.0 software.
Ethical approval
The study was approved by the Ethics Committee of Meir Medical Center (protocol number 087-2010). Written informed consent was obtained from all participants. The NIH study number is NCT01167647
Results
Patient characteristics
The study group included 155 patients evaluated by bronchoscopy for suspected malignancy. The cohort included 102 men (66%) and 53 women (34%) at a mean age of 68±11 years. More than half of the patients 92 (59%) had previous malignancy, 54 (35%) presented with hemoptysis and 6 (4%) had underlying lung disease. The most prominent radiologic finding on HRCT was lung mass in 106 (68%) followed by lung infiltrates in 23 patients (15%). The majority of patients were diagnosed with cancer (60%). In total, only 21 of the 155 patients presented with clinical signs of infection (Table 1). Berghmans et al. found that the distribution of the principal types of infection was similar irrespective of the histology [non-small cell lung cancer (NSCLC) vs. small cell lung cancer (SCLC)] or disease extent (NSCLC) (9). Therefore, SCLC patients (5%) were also included in the study, and were added to the ‘cancer’ group.
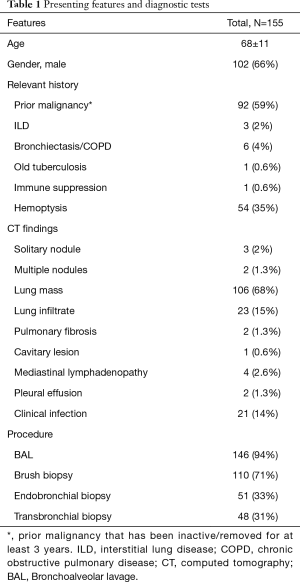
Full table
The rate of positive PPM cultures supports the need for routine bacterial sampling
Positive bacterial cultures with PPM’s were demonstrated in 46 patients (30%) of the entire cohort (Table 2). The rate was similar for those with and without a cancer diagnosis (Table 2). We found that PPM growth was not significantly associated with the presence of centrally-located endobronchial lesions (56.5% vs. 49.5%, P=0.131).

Full table
As expected, the presence of clinical features of infection was the only baseline characteristic predictive of significant bacterial growth with PPM, 35% vs. 5%, respectively (P<0.001). Table 2 demonstrates the relationship between significant bacterial growth and clinical features of infection in cancer and non-cancer patients. Overall, the distribution of clinical signs of infection was similar in both groups, as well as the rate of the positive PPM cultures (~30%). Interestingly, more than half (30/46) of the positive PPM samples were taken from patients without any clinical signs of infection.
These findings suggest that routine bacterial cultures should be recommended, especially for the lung cancer patients which are at increased risk to develop pulmonary complications.
PPM distribution
In line with our other observations, among those with positive bacterial growth the rates of gram negative organisms and gram positive were similar for patients with and without cancer. The most frequently isolated organisms were Pseudomonas sp. followed by Staphylococcus aureus (Table 3). In their work, Lee et al. (5) found that malignant tissues contained more Firmicutes in comparison to benign tissue. However, we did not observe these changes in our cohort.
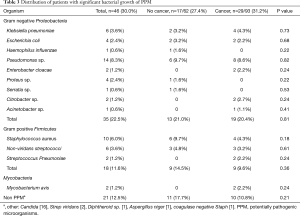
Full table
Discussion
Diagnosis of bacterial colonization of the airways is usually clinically important. In patients with lung cancer awaiting potentially curative resection, surgery may be delayed due to clinical or radiologic signs of infection (11). Bacterial colonization of the airways demonstrated intra-operatively is associated with an increased rate of post-operative complications including POP (8,14) leading to increased morbidity and mortality (11,12).
In this prospective study, we tested the growth of PPMs in 155 consecutive patients with suspected lung cancer evaluated by bronchoscopy in our center. Our main goal was to test whether the ‘traditional’ microbiology methods would distinguish between lung cancer and those with benign mass-like lesions. In addition, we evaluated the rate of potentially undiagnosed patients that would benefit from antibiotic treatment to prevent POP and other complications.
In our study, we compared patients eventually diagnosed with cancer vs. patients with non-malignant lesions. We found that PPM growth was not significantly associated with cancer diagnosis. In a report by Ioanas et al. (10) analyzing tissue samples and not bronchoalveolar lavage (BAL), central location of tumor was associated with bronchial colonization among 41% of the patients with resectable cancer. In a study by Cabello et al., samples were obtained by both BAL and protected specimen brush (PSB) from 33 carcinoma patients. In fact, the colonization rate was higher when samples were obtained by the PSB. Thus, referring to the collection method is important when comparing data from different cohorts.
Our study only included patients with suspected lung cancer undergoing bronchoscopy, and thus did not include a healthy patient group. A study that comparing BAL samples from 15 healthy and 28 bronchogenic carcinoma patients found that only 4 patients were colonized (25%), mostly by non-PPM (17). Moreover, although some microorganisms were found amongst the carcinoma group, the healthy patients were mostly sterile (87%). Interestingly, the ‘non-cancer’ group in our study showed more colonization than the previously reported ‘Healthy’ by Cabello et al. (17). These findings suggest that although the diagnosis was eventually benign, the colonization PPM rate was higher. These results warrant further study.
Overall, significant bacterial growth of PPMs was observed in 30% of our patients. These results resemble previous studies that reported the development of POP among 31% of patients with lung cancer undergoing lung resection (8). In their study, Belda et al. only tested lung cancer patients (N=78) undergoing surgery, thus not referring to healthy/non-malignant lesions. In addition, a number of groups reported that POP significantly correlated with perioperative bronchial colonization and associated with longer hospitalization and ICU stay (8,12,14,18,19). Interestingly, they also found a correlation between positive PPM culture and postoperative clinical infection, resembling our results.
Pulmonary infections are an important complication of lung cancer patients undergoing medical treatment. In a report by Hansel et al., 11/276 patients with SCLC developed lung abscesses within 1 month of initiation of chemotherapy and demonstrated shorter median survival (182 vs. 224 days, not significant) (20).
Berghmans et al. prospectively studied 275 lung cancer patients hospitalized for any cause with 435 episodes of fever and/or other documented infection (9). The tracheobronchial tree was the predominant site of infection (56%) among which H. influenzae, S. pneumoniae, Pseudomonas aeruginosa, E. coli and M catarrhalis were the predominant pathogens, each responsible for approximately 10% of documented micro-organisms (9). In our study, Pseudomonas aeruginosa and Staphylococcus aureus were the predominantly isolated PPM, but S. pneumonia, E.coli and H. influenza were identified as well.
Unlike the NGS study results that showed that differences exist in the bacterial communities of patients with lung cancer and those with benign mass-like lesions (5), our results were similar in both groups. In our study, the most commonly isolated pathogenic organisms included Pseudomonas sp., Klebsiella pneumoniae, Staphylococcus aureus and Enterobacteriaceae. These results are similar to previous studies of perioperative bronchial colonization among lung cancer patients undergoing lung resection (8,10-12). Furthermore, concordance between the pathogen responsible for colonization and POP could be proven in 85% of those with positive intraoperative aspirates (14). Thus, emphasizing the importance of diagnosing pulmonary infections preoperatively.
In the current study, bronchial colonization with PPM was demonstrated among lung cancer patients at diagnosis. Of the 46 patients with positive PPM growth, only 16 presented with clinical signs of infection. Therefore, the rate of potentially undiagnosed pulmonary infections was 65% (30/46). In agreement, as shown by Schussler et al., modification of antibiotic prophylaxis to target predominant bronchial organisms using short time regimen decreased the incidence of POP by 39.5% (14). Therefore, they suggested that a short-time targeted antibiotic prophylaxis against bacteria that colonize the bronchi of patients at the time of operation for major lung resection may be crucial in the prevention of POP.
In conclusion, although bronchoscopy is routinely applied for patients with a suspicion of lung cancer, microbiological culture from BAL may not be essential.
Since most of the patients with clinical signs of infection will demonstrate significant PPM growth, these patients should be treated based on the existence of clinical infection.
However, additional studies are suggested to further examine the clinical impact of early, targeted antibiotic treatment among patients who are candidates for potential curative lung resection or those treated medically.
Acknowledgements
We thank Dr. Nira Koren for assistance with the statistical analysis and Faye Schreiber for editorial support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of Meir Medical Center (protocol number 087-2010). Written informed consent was obtained from all participants.
References
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 2012;336:1268-73. [Crossref] [PubMed]
- Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013;14:207-15. [Crossref] [PubMed]
- Chng KR, Chan SH, Ng AH, et al. Tissue Microbiome Profiling Identifies an Enrichment of Specific Enteric Bacteria in Opisthorchis viverrini Associated Cholangiocarcinoma. EBioMedicine 2016;8:195-202. [Crossref] [PubMed]
- Karin M, Jobin C, Balkwill F. Chemotherapy, immunity and microbiota--a new triumvirate? Nat Med 2014;20:126-7. [Crossref] [PubMed]
- Lee SH, Sung JY, Yong D, et al. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer 2016;102:89-95. [Crossref] [PubMed]
- Shitrit D, Vertenshtein T, Shitrit AB, et al. The role of routine culture for tuberculosis during bronchoscopy in a nonendemic area: analysis of 300 cases and review of the literature. Am J Infect Control 2005;33:602-5. [Crossref] [PubMed]
- Talker O, Matveychuk A, Guber A, et al. Is routine bronchoscopic culture indicated in areas with low tuberculosis prevalence? Int J Tuberc Lung Dis 2013;17:1100-3. [Crossref] [PubMed]
- Belda J, Cavalcanti M, Ferrer M, et al. Bronchial colonization and postoperative respiratory infections in patients undergoing lung cancer surgery. Chest 2005;128:1571-9. [Crossref] [PubMed]
- Berghmans T, Sculier JP, Klastersky J. A prospective study of infections in lung cancer patients admitted to the hospital. Chest 2003;124:114-20. [Crossref] [PubMed]
- Ioanas M, Angrill J, Baldo X, et al. Bronchial bacterial colonization in patients with resectable lung carcinoma. Eur Respir J 2002;19:326-32. [Crossref] [PubMed]
- Schussler O, Alifano M, Dermine H, et al. Postoperative pneumonia after major lung resection. Am J Respir Crit Care Med 2006;173:1161-9. [Crossref] [PubMed]
- Stephan F, Boucheseiche S, Hollande J, et al. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest 2000;118:1263-70. [Crossref] [PubMed]
- Vento S, Cainelli F, Temesgen Z. Lung infections after cancer chemotherapy. Lancet Oncol 2008;9:982-92. [Crossref] [PubMed]
- Schussler O, Dermine H, Alifano M, et al. Should we change antibiotic prophylaxis for lung surgery? Postoperative pneumonia is the critical issue. Ann Thorac Surg 2008;86:1727-33. [Crossref] [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27-72. [Crossref] [PubMed]
- Ballows A, Harsler WJ. Manual of Clinical Microbiology. 5th edition. Washington, DC: American Society for Microbiology, 1991.
- Cabello H, Torres A, Celis R, et al. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: a bronchoscopic study. Eur Respir J 1997;10:1137-44. [Crossref] [PubMed]
- Putinati S, Trevisani L, Gualandi M, et al. Pulmonary infections in lung cancer patients at diagnosis. Lung Cancer 1994;11:243-9. [Crossref] [PubMed]
- Qiao D, Wang Z, Lu Y, et al. A retrospective study of risk and prognostic factors in relation to lower respiratory tract infection in elderly lung cancer patients. Am J Cancer Res 2014;5:423-32. [PubMed]
- Hansen SW, Aabo K, Osterlind K. Lung abscess in small cell carcinoma of the lung during chemotherapy and corticosteroids: an analysis of 276 consecutive patients. Eur J Respir Dis 1986;68:7-11. [PubMed]


