Minimally invasive aortic valve replacement surgery through lower half sternotomy
Introduction
There are multiple factors that come to bear when considering a less invasive approach for aortic valve surgery. Any alternative operation should have equivalent outcomes to the standard sternotomy approach. At a minimum, this means there is no compromise to safety and implantation of the ideal prosthesis for each individual patient. Secondary factors such as accelerated physical recovery, reduced postoperative pain, reduced incidence of transfusion, shorter ICU and total hospital stay, and cosmetic result are important but should not compromise the main objectives of the operation (1-3). At no point should market factors become the primary driving force for a less invasive operation.
Minimally invasive approach
Success with a minimally invasive approach requires accurate knowledge of each patient’s specific anatomic and pathologic characteristics, flexibility on the part of the surgeon, and a well-devised incision. The ideal minimally invasive incision would allow for access to all necessary areas of the heart, use no or minimal extra or specialized equipment, provide excellent visualization, and most importantly, allow for the identical operation to be performed as would be using the standard approach.
A variety of incisions have been described for minimally invasive aortic valve surgery, including anterior thoracotomy, parasternal incision, transverse sternotomy, and partial/limited upper sternotomy. Each of these approaches has specific drawbacks, such as universal need for peripheral cannulation, lung herniation, poor sternal healing, risk of injury to the internal mammary artery, or expensive unique instrumentation, to name just a few (4-6). Conversion of some of these incisions to a full sternotomy is not infrequent, particularly with the upper partial sternotomy approach.
The lower partial sternotomy is a versatile operation that provides direct access to the aortic root (7,8). In the vast majority of patients, familiar techniques of cardiopulmonary bypass, myocardial protection, and valve implantation are maintained. Through this incision, all the surfaces of the heart can be exposed and the potential benefits of a minimally invasive operation achieved.
Surgical technique
Incision and exposure
With the patient supine and anesthetized, inspection of the thoracic wall is undertaken. The angle of Louis defines the second rib and the second and third interspace is identified. A vertical skin incision 8-10 cm in length is placed in the midline over the sternum and extended inferiorly starting from the third intercostal space (Figure 1).

The pectoralis major and intercostal muscles are separated laterally from the sternum at the third interspace for a short distance to free the internal mammary artery pedicles. The sternum is divided transversely at the level of the third interspace and then vertically in the midline from that point inferiorly through the xiphoid using a standard sternal saw, leaving the upper half of the sternum intact (Figure 1).
A small Kuyper-Murphy retractor (Canadian type) with concave blades 3 inches (7.6 cm) wide (Pilling-Weck Co, Ft. Washington, PA) is used to separate the lower sternal edges. In some patients, this may elevate the lower sternal edges above the intact upper sternum and obscure the underlying structures, especially the ascending aorta. In this situation, a Cheanvechai-Favaloro Internal Mammary Retractor with the Cheanvechai Swivel Rake Assembly (sharpened) is used to elevate the intact upper sternum anteriorly and superiorly (Figure 2).
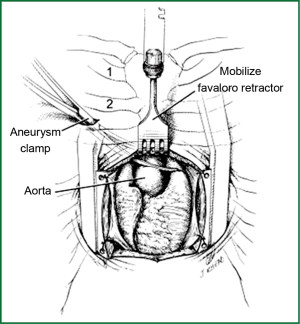
As the intact upper sternum is elevated from the underlying mediastinal structures, the exposure “grows” to the familiar median sternotomy approach, despite the small skin and sternal incisions. A longitudinal pericardial incision is made, and retraction stitches are placed on the edges of the pericardial sac to elevate the heart anteriorly. Additional exposure can be obtained by opening the right pleural space and also by removing the thymus gland.
Cannulation and cardiopulmonary bypass
Aortic cannulation is as high on the ascending aorta as possible, ideally near the pericardial reflection, even though this is beneath the intact upper sternum. The usual double pursestrings are placed to accommodate the inflow cannula. A 22 or 24F thin-walled, wire-reinforced perfusion cannula is employed (EOPA 3D Cannula; Medtronic Co, Minneapolis, MN). This cannula is small and flexible and can be introduced directly through the sternotomy incision and secured unobtrusively to the chest wall at the upper end of the incision. This cannula is inserted using a needle-guidewire technique, which allows for safe and controlled cannulation.
Venous cannulation is by either a single two-stage cannula (29 to 37F thin wall, MC2 Venous Cannula; Medtronic Co, Minneapolis MN) or a double-cannula bicaval technique (24F thin wall; Baxter Research Medical). These are small, flexible cannulae that can be brought directly out through the primary incision. An alternative method for bicaval cannulation uses separate stab incisions in the chest wall which later become the exit sites for the thoracostomy drains. In this technique, the superior vena cava cannula enters the right hemithorax through a stab incision on the chest wall above the right costal margin. This cannula is brought across the open right pleural space to enter the wall of the right atrium and then advanced into the superior vena cava. The inferior vena cava right-angled cannula enters through a stab incision slightly to the right of the midline below the costal margin, into the right atrium, and is advanced into the inferior vena cava. Venous drainage may also be accomplished peripherally via the right internal jugular vein by with a 16F thin-walled, wire-reinforced cannula (SPC 2538, TF-024 with long tip and multiple holes; Baxter Research Medical) combined with a 25F femoral venous cannula (QuickDraw venous cannula, Edwards) by percutaneous needle guidewire technique. Active venous uptake using vacuum drainage is universally employed for these operations.
A standard left ventricular vent catheter is introduced through the right superior pulmonary vein and exits either through the primary incision or is brought out through the right pleural space via a separate stab incision. Perfusion catheters for retrograde or antegrade cardioplegia are small enough to be placed through the primary incision.
To cross-clamp the aorta, a stab incision is made on the anterior chest wall on the right side below the clavicle, and a 10-inch (26.4 cm) or 12-inch (31.8 cm)-long DeBakey aortic aneurysm clamp (Pilling-Weck) is passed into the open right pleural space. The length of the clamp is chosen to place the hinge of the device in the chest wall with the jaws of the clamp across the ascending aorta (Figure 2). The aorta is then occluded in an anterior-posterior manner as close to the inflow cannula as possible, with the posterior blade of the clamp in the transverse sinus. This position provides the greatest length of ascending aorta for manipulation.
Aortic valve replacement
Complete transection of the aorta just above the sinotubular junction provides superior exposure of the aortic root and subvalvular left ventricular outflow tract. In addition, the lateral position of the cross clamp flattens the posterior wall of the aorta, making closure of the aorta straightforward following valve replacement. Following the valve operation, de-airing is performed in the usual manner after closure of the cardiac incisions.
Closure
Following separation from cardiopulmonary bypass and removal of cannulae, thoracostomy drains are brought out through previously placed stab incisions used for venous uptake or vent cannulas. Sternal closure is accomplished with two #6 sternal wires placed between the divided portion of the sternum and the intact sternum above on the left and right sides. These wires are crossed anterior to the sternum and tied to the opposing wire. The effect is to tightly secure the sternal edges at the T as the two wire knots are twisted down. Three or four parasternal wires are placed to secure the divided lower sternum.
Contraindications and special considerations
This technique is suitable for nearly every patient and so there are no absolute contraindications. If the patient body habitus is significantly obese or the sternum is short, the superior extent of the sternotomy can be extended to the second interspace. This slightly higher sternal division can facilitate the operation in terms of visualization and also allow for more direct cardiac palpation if necessary. Preoperative evaluation with plain chest radiographs or computed tomography is useful for anatomic planning to place the incision directly over the aortic valve and ascending aorta.
It is usually necessary to use instruments 2.5 cm longer than are usually employed, but extra-long or custom instruments are seldom required. Sutures are typically tied manually; a knot pusher or autosuturing device (Cor-Knot, LSI Solutions Inc, Victor, NY) can be used to secure especially deep sutures. Internal defibrillation is performed with standard or pediatric paddles, depending on the limits of the patient anatomy.
Outcomes
To date, there are no prospective randomized clinical trials that can answer the question of superiority of one approach over another. The successful use of the lower half sternotomy technique has been well described and clinical outcomes are equivalent to standard sternotomy (3,7,8). In our series, there was a zero rate of conversion to a full sternotomy, and other complication rates were comparable to the standard full sternotomy approach (7,8). Operative time has been shown to consistently decrease as the surgeon and operative team became more familiar with the procedure (5). This “learning curve” is probably shorter than with other, more complicated minimally invasive operations. All forms of aortic valve operations can be performed via this approach, including stented valves, stentless valves, aortic root replacement, and even pulmonary autografting (Ross procedure). Finally, short term pain levels, ICU length of stay, and overall hospital length of stay were all improved or shorter with the lower partial sternotomy, indicating a quicker functional recovery from this surgery.
Conclusions
Minimally invasive aortic valve surgery presents a unique set of challenges and opportunities to the practicing cardiac surgeon. When performed through a lower half sternotomy approach, an identical operation with only minor adjustments can be performed with similar outcomes. Benefits include improved cosmesis, reduced short-term pain level and quicker overall recovery. This approach should be considered whenever a minimally invasive aortic valve operation is indicated and used with appropriate clinical judgment and experience.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Svensson LG, Atik FA, Cosgrove DM, et al. Minimally invasive versus conventional mitral valve surgery: a propensity-matched comparison. J Thorac Cardiovasc Surg 2010;139:926-32.e1-2.
- Mihaljevic T, Cohn LH, Unic D, et al. One thousand minimally invasive valve operations: early and late results. Ann Surg 2004;240:529-34; discussion 534. [PubMed]
- Hsiao CY, Ou-Yang CP, Huang CH. Less invasive cardiac surgery via partial sternotomy. J Chin Med Assoc 2012;75:630-4. [PubMed]
- Arom KV, Emery RW, Kshettry VR, et al. Comparison between port-access and less invasive valve surgery. Ann Thorac Surg 1999;68:1525-8. [PubMed]
- Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7. [PubMed]
- Brown ML, McKellar SH, Sundt TM, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670-679.e5.
- Doty DB, DiRusso GB, Doty JR. Full-spectrum cardiac surgery through a minimal incision: mini-sternotomy (lower half) technique. Ann Thorac Surg 1998;65:573-7. [PubMed]
- Doty DB, Flores JH, Doty JR. Cardiac valve operations using a partial sternotomy (lower half) technique. J Card Surg 2000;15:35-42. [PubMed]


