Minimally invasive mitral surgery through right mini-thoracotomy under direct vision
Introduction
In the 1980s, Carpentier proposed an organizational framework of evaluation and reparative techniques for mitral valve (MV) insufficiency as an alternative to valve replacement. His series of 551 patients who underwent mitral valve repair (MVr) demonstrated that predictable and stable long-term results could be achieved with a low incidence of thromboembolism (1,2). Our extensive clinical experience in MVr at NYU was pioneered by Dr. Stephen Colvin, who visited Carpentier in 1978. The initial NYU results from 1980-1985 were reported in 1985 (3), and subsequent follow-up results on 148 patients were reported in 1988 (4). The mortality was 1.2% and the five years survival from cardiac death or reoperation was 90%, with a 92.3% freedom from significant (3-4+) recurrent mitral regurgitation. In 1989 we reported that the patients receiving MVr had less valve-related combined morbidity than patients receiving valve replacement (5). Others throughout Europe and the U.S.A. were simultaneously reporting similar findings and MVr progressively became the preferred treatment for patients with MV insufficiency.
In the early 1990s the success of laparoscopic operations in general surgery sparked a new interest in minimally-invasive approaches for cardiac surgery. Limited anterior thoracotomy, also called minimally invasive direct coronary artery bypass (MIDCAB), was explored in Europe and the U.S.A. for off pump mammary artery to left anterior descending artery coronary bypass. With these approaches under development, Dr. John Stevens from Stanford University and Dr. Wesley Sterman founded Stanford Surgical Technologies, a company eventually renamed Heartport. This entity developed technology to facilitate on-pump arrested heart coronary bypass and valve surgery through a mini-thoracotomy, termed the “Port-Access” approach. Their innovations included balloon endoaortic occlusion technology, specialized retractors, and other newly designed instruments designed for minimal access cardiac surgery.
In 1994 Dr. Greg Ribakove, a NYU faculty member on sabbatical at Stanford, began preclinical work with Stevens, the Heartport Company, the Stanford research team, and our NYU laboratory to further develop these technologies for clinical use. Animal studies in our laboratory demonstrated that the right anterior mini-thoracotomy technique was technically reproducible, achieved normal mitral valve placement, and resulted in complete cardiac functional recovery (6), with similar feasibility studies demonstrated by the Stanford group. These studies led to initial clinical trials with minimally invasive “Port-Access” cardiac surgery using balloon endoclamp and an arrested heart. The first human case for right anterior thoracotomy MV surgery with an endoclamp was performed in March 1996 in Malaysia by the Stanford team of Tom Burdon and Mario Pompili. Three months later Mohr and colleagues in Leipzig also began using the endoclamp technology for performing MVr through a right anterior thoracotomy (7). Despite concerns over a potential increased risk of stroke or aortic dissection, the initial results were encouraging.
In October 1996, Stevens and colleagues at Stanford University and Colvin and colleagues at NYU launched the FDA phase I clinical trial for the Port-Access system in the U.S.A. Initial NYU results included 151 patients, 113 of whom underwent MVr through a right anterior mini-thoracotomy (8). A report from the Port-Access multicenter registry suggested that this approach was safe and effective (9). Simultaneously Navia and Cosgrove (10) and Cohn et al. (11) reported minimally invasive MVr using right para-sternal approaches with direct aortic crossclamping. All of these experiences fueled expectations that by avoiding a conventional sternotomy, safe and reproducible results could be achieved with minimally invasive valve surgery, while minimizing morbidity, pain, blood loss, and hospital length of stay.
Technique
Incisional approach
At our institution the current preferred approach for right mini-thoracotomy MV operation is through the 3rd or 4th interspace mini-thoracotomy incision. In men, a 3rd interspace approach allows both excellent mitral viewing and easy access to the aorta for direct cannulation, cardioplegia/venting needle placement, and external cross-clamping. In women, our preferred approach is placing the skin incision below the infra-mammary fold with a 4th interspace incision. This approach provides cosmesis for women with a direct view from a lateral perspective into the left atrium and MV (12). Accessing the aorta can be more challenging through the lower interspace incision; direct cannulation can be facilitated with high tech cannulae such as the StraightShot® aortic cannula (Cardiovations, Edwards Life-sciences, Irvine, CA) or EasyFlowTMEstech cannula (Estech, San Ramon, CA) or alternately the surgeon may use femoral arterial perfusion in patients without extensive vascular disease.
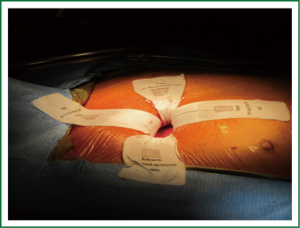
Typically a skin incision of 5 to 6 cm in length is created over the chosen interspace with the intercostal incision being extended beyond the limits of the skin incision. This allows for a retractor to spread the ribs while minimizing the risk of breaking them. The skin and underlying intercostal incisions can be enlarged depending on patient body habitus and the experience of the surgeon. A soft tissue retractor is placed into the wound to prevent tissue debris from adhering to the sutures as they are passed in and out (Figure 1). A suture is at times placed through the dome of the right hemi-diaphragm if it is high and the ends are withdrawn inferiorly through what will later become a tube thoracostomy site. This maneuver is particularly useful in 4th interspace approaches where the hemi-diaphragm can obstruct most of the lateral pericardium.
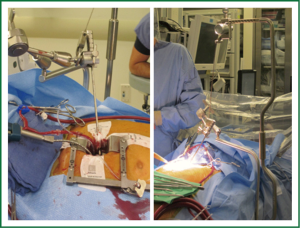
The pericardium is opened anterior to the phrenic nerve; care is taken to avoid the nerve so as to help minimize the risk for stretch injury. Multiple pericardial retention sutures are placed anteriorly and posteriorly to retract and define the access to the Sondergaard’s groove and access to the aorta. After completion of cannulation and with the patient on cardiopulmonary bypass, cardioplegic arrest is obtained and the groove opened. A floppy vent cardiotomy sucker is dropped into the open left pulmonary veins and a retractor is placed to elevate the inter-atrial septum exposing the MV. The exteriorized handle of the retractor is connected to an “iron intern” system to provide external constant elevation without compression of the chest wall (Figure 2). Care is taken not to exert excessive retraction; this can kink or dislodge a portion of the venous cannula in the superior vena cava (SVC) causing an SVC syndrome. We typically monitor the pressures in the SVC to verify that this is not occurring.
Direct visualization with this operation is typically excellent. Depending on body habitus, the surgeon will choose either short (15 cm) or long (22 cm) minimally invasive instruments. These tools are critical; their low-profile handles are necessary to prevent the surgeon’s hands from blocking his/her view (Figure 3). Facilitation with a knot pusher is also imperative. Sometimes in deep chested patients it can be slightly difficult to visualize the entire mitral annuls, especially the anterior annulus. In such case the annular sutures are placed first to facilitate exposure of the leaflets and entire annulus.
Aortic cannulation and perfusion
Our original approach to the right mini-thoracotomy utilized a classic Port-Access technique (13). This consisted of retrograde arterial femoral perfusion, long cannula femoral venous drainage, and retrograde cardioplegia via a coronary sinus catheter placed by anesthetist through an internal jugular line. Aortic cross-clamping, antegrade cardioplegia administration, and aortic root venting was accomplished with an endoclamp placed through a side-limb of the femoral arterial cannula. The endoclamp is a multi-lumen catheter with an inflatable balloon at its distal end which provides endo-aortic clamping. A central lumen can provide antegrade cardioplegia delivery or alternatively aortic root venting. A second tip lumen allows monitoring of aortic root pressure (14). A small but significant risk with retrograde perfusion is that of aortic dissection, particularly in those unscreened patients with aortic and peripheral vascular disease. Because of this issue and the increased risk of retrograde atheromatous embolization in diseased arterial vasculatures, our right mini-thoracotomy approach has evolved into a simpler technique with direct ascending aortic cannulation if extensive vascular disease is present (15). We continue to utilize femoral cannulation with a #18 or #20 straight cannula in select patients without extensive vascular disease, but with no “endoclamp” and with direct aortic crossclamping with a flexible crossclamp. The retrograde perfusion and endoclamp approach is currently used only for our robotic MVrs, where a thorough vascular angiographic evaluation is conducted preoperatively.
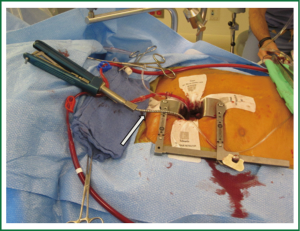
Our current standard approach through a right mini-thoracotomy includes a trans-incision placement of aortic purse string sutures and direct aortic cannulation. If direct aortic cannulation is utilized we typically use a standard #20 straight aortic cannula (Edwards Life-sciences, Irvine) while a StraightShot® aortic cannula (Cardiovations, Edwards Life-sciences, Irvine, CA) or EasyFlowTMEstech aortic cannula (Estech, San Ramon, CA) can facilitate direct cannulation of ‘distant’ aortas. This cannula can even be placed through a separate thoracoscopic port but we would never advocate cannulation of an aorta where the surgeon could not apply direct and immediate finger pressure for control of potential bleeding. Our perfusion system is completed with long femoral venous cannula drainage, often placed percutaneously over a guidewire, using echocardiographic guidance to position the cannula in the right atrium, with the tip in the SVC. An antegrade cardioplegia and vent needle is placed in the ascending aorta and if necessary a retrograde cardioplegia catheter is placed trans-atrialy in the coronary sinus under TEE guidance. After initiating cardiopulmonary bypass direct external aortic clamping is achieved using a flexible crossclamp (Figure 4). By placing the coronary sinus catheter directly through the right atrium, the demands on the anesthesia team and costs are reduced (16). We have demonstrated that this approach of direct aortic cannulation and external clamping is associated with a low risk of neurologic complication and good clinical outcomes (15).
Indications and contraindications
For some patients, the above approach may have relative contraindications. The contraindications to right mini-thoracotomy MVr are those conditions associated with poor exposure. In patients with prior right lung surgery, resection, or pleurodesis it will be difficult or near impossible to create an appropriate thoracic workspace. Additionally, obtaining adequate exposure can be difficult in the morbidly obese patient and in those patients with significant chest wall abnormalities such as pectus excavatum or those with unusual cardiac orientations (17).
For a small subset of patients with multiple prior open procedures or mediastinal muscle flaps an alternative approach to the MV is through a left posterior mini-thoracotomy (18). The left-sided approach also offers some advantages, including a wider angle of vision and a shorter distance from the surgeon to the MV (19). A larger left postero-lateral thoracotomy incision is made, the pericardium is opened anterior to the phrenic nerve and an incision is made parallel to the atrioventricular groove in the left atrium, frequently excising the base of the left atrial appendage. Most are done fibrillating, with direct cannulation of the descending aorta and percutaneous long venous cannulation. Of course the mitral apparatus is reversed for the surgeon as compared to the standard access. This left sided technique is an excellent approach for the mutli-reoperative MV for replacement or paravalvular leak correction. This approach is most favorable for valve replacement and not repair; repair can prove difficult as the surgeon does not always have a good view of the subvalvular apparatus.
Repair
As mentioned previously, MVr is performed under direct vision using long, low-profile surgical instruments. Posterior and anterior leaflet pathology from degenerative valve disease is repaired using standard techniques and an annuloplasty band or ring is placed in all of these patients to reinforce the repair and remodel the annulus to the correct size and shape. The repair techniques are identical to those used routinely with a traditional sternotomy approach.
Outcomes
The NYU experience with the right anterior mini-thoracotomy approach
Between 1995 and 1997, 151 minimally invasive valve procedures were performed at NYU using the Heartport Port-Access technology system (Cardiovations, Edwards Life-sciences, Irvine, CA). The operative mortality rate for isolated minimally invasive MV surgery was 1.1% and for all minimally invasive MV surgery was 3.5% in this series (13).
Subsequently from 1996-1998, 109 consecutive patients undergoing Port-Access isolated valve surgery (aortic and mitral) were compared with 88 matched patients who underwent isolated valve surgery via a median sternotomy. Analysis revealed that the Port-Access minimally invasive approach was associated with similar hospital mortality rates, shorter length of stay, fewer transfusions, and fewer septic complications than the sternotomy group (20). Similarly, outcomes from our first 100 consecutive isolated Port-Access minimally invasive MVr cases were compared with those from our previous 100 patients undergoing primary MVr through the standard sternotomy approach. There was one hospital death in the sternotomy group and none with the minimally invasive group (P= NS). At one year follow-up MR and freedom from reoperation were similar between the two groups, while follow-up New York Heart Association class was significantly better in the patients undergoing the minimally invasive approach (1.5 vs. 1.2) (21).
By 2001, 714 patients had undergone minimally invasive MV operations at our institution-561 of which were isolated MV operations with 66.8% being MVr and 33.2% mitral replacements. Arterial cannulation was femoral in 79% using the Port-Access endoclamp and central in 21%. Cardioplegia was either trans-jugular retrograde, direct retrograde, or antegrade. Right anterior mini-thoracotomy was used in 96.6% and left posterior mini-thoracotomy in 2.2%. Hospital mortality for primary isolated minimally invasive MVr was 1.1% and multivariate analysis demonstrated that previous cardiac operation, New York Heart Association functional class IV, advanced age, mitral replacement, and an emergency operation were significantly associated with increased operative risk. Major complications totaled 6.6% overall, including reoperation for bleeding (4.9%), reoperation for valve failure (<1%), aortic dissection (<1%), neurologic deficit (2.9%), sepsis (2.9%), renal failure (2.2%), respiratory failure (7.1%), mediastinal infection (0%), and chest wall infection (<1%). Follow-up echocardiography demonstrated 89.1% of the MVr patients had only trace or no MR (22).
During that same time we analyzed results from minimally invasive MVr in re-operative patients. Four hundred ninety-eight patients with previous cardiac operations via sternotomy underwent isolated valve surgery: 337 (117 mitral) via median sternotomy and 161 (100 mitral) via right anterior mini-thoracotomy. Preoperative incidences of congestive heart failure, renal disease, and non-elective procedures were higher in the sternotomy group. Hospital mortality was significantly lower with minimally invasive MVr, 5% as compared to 11.3% in the sternotomy group. Mean bypass time, cross-clamp times, and stroke rates were the same in these two groups. In the minimally invasive group there were no deep wound infections, less need for blood products, and shorter hospital length of stay. Five-year survival was higher with the minimally invasive approach as compared to sternotomy (92.4% vs. 86%). Based on these results we believe that for re-operative MV surgery, a minimally invasive approach can be safely performed with at lower morbidity and mortality, decreased length of stay, and favorable mid-term survival as compared to a median sternotomy approach (23).
More recently we reported our experience with mitral valve repair in more than 3,000 patients between 1986 and 2008. Of these, 1,071 patients with degenerative disease had MVr performed through a right mini-thoracotomy and direct vision [1996-2008]; while 530 patients had traditional sternotomy. The overall operative mortality was 1.3% for patients having isolated mitral valve repair for both the standard sternotomy and the right anterior mini-thoracotomy approaches (NS), and likewise there was no difference in the incidence of perioperative stroke (NS). The 8-year freedom from reoperation was 91% for the sternotomy group and 95% for the right anterior mini-thoracotomy and the 8-year freedom from reoperation or severe (3+, 4+) recurrent MR was 90% for sternotomy and 93% for right anterior mini-thoracotomy. Eight-year freedom from all valve-related complications was 86% for the sternotomy patients and 90% for right anterior mini-thoracotomy patients (24).
Overall at NYU we have collected prospective data on over 4,000 MVr patients over 35 years, 1,922 of which were performed through a right anterior mini-thoracotomy. With the mini-thoracotomy approach the risks of perioperative death and stroke have been demonstrated to be equivalent to those achieved with the standard sternotomy, while hospital stay, risk of bleeding and the need for transfusion have been less with mini-thoracotomy. Long term results in terms of freedom from recurrent mitral insufficiency have also been shown to be equivalent to those achieved with sternotomy. Consequently, the right mini-thoracotomy approach has therefore become our technique of choice for isolated mitral valve repair.
Other centers’ experience
Seeburger et al. from Leipzig reported outcomes for a series of 1,536 consecutive patients who underwent minimally invasive MV surgery for MR through a right mini-thoracotomy. 87.2% of these patients underwent MVr with a 0.3% conversion rate to sternotomy. Pre-discharge echocardiography showed a mean MR grade of 0.2. Thirty-day mortality was 2.4%, five years survival was 82.6%, and freedom from MV reoperation was 96.3%, demonstrating a safe and durable MVr (25). This group also compared outcomes after MVr through right mini-thoracotomy versus median sternotomy in patients greater than 70 years old. 1,027 patients greater than 70 years old underwent isolated MVr between 1999 and 2009 and were analyzed for outcome differences using propensity score matching. They performed a right mini-thoracotomy with femoral cannulation and trans-thoracic aortic cross-clamping in the majority of the minimally invasive group. There were no differences between the matched groups in 30-day mortality, long term survival, major adverse cardiac and cerebrovascular events, or other adverse events. Based on their findings they concluded that minimally invasive MV surgery through a right mini-thoracotomy is equivalent and as safe as the sternotomy approach in elderly patients (26).
Gammie et al. have a series of 187 patients on which they performed MV surgery through a right mini-thoracotomy. The rate of MV repair was 96.3% and was 100% in patients with degenerative disease. There were no deaths, strokes, renal failure, or wound infections. Two patients were re-explored for bleeding and 27% of patients received blood transfusions. The median hospital stay was 4 days. Freedom from significant MR at hospital discharge was 99% and survival at a median follow-up of 2.5 years was 99%, again demonstrating a safe and durable MVr through a right mini-thoracotomy (27).
A report by D’Alfonso et al. analyzes a single-surgeon experience with MV surgery through a right mini-thoracotomy with peripheral cannulation and external aortic cross-clamping. Between 1999 and 2010, 179 patients underwent MVr. There were no in-hospital deaths and all patients were discharged with less than 2+ MR. At ten years’ follow-up, overall survival was 98.7%, freedom from reoperation was 98.5%, and freedom from MR recurrence was greater than 90% (28).
The group at University of Pennsylvania examined the outcomes of MVr through right mini-thoracotomy versus median sternotomy in 1,011 isolated MVrs. A right mini-thoracotomy approach was used in 66% and sternotomy in 44%. Propensity scores identified 201 well-matched pairs with MR of any cause and 153 pairs with myxomatous MV disease. In-hospital mortality, incidence of stroke, infection, myocardial infarction, exploration for postoperative hemorrhage, renal failure, and atrial fibrillation were comparable between the two groups. Over nine years of follow-up, there was no significant difference in long-term survival between the two groups. There were fewer transfusions and fewer early readmissions in the right mini-thoracotomy group. The authors concluded that this study affirms the non-inferiority of MV surgery via right mini-thoracotomy as compared to median sternotomy (29).
Mohr et al. examined a series of 39 patients who underwent redo MV surgery through a right mini-thoracotomy using the Port-Access technique and videoscopic assistance. In all cases, femoro-femoral cannulation was performed. These patients were compared to a retrospective group of 25 patients who underwent redo MV surgery via a median sternotomy. Time of surgery and cross-clamp time were comparable between the two groups. Mortality in the minimally invasive group was 5.1% and one patient had transient hemiplegia due to the migration of the endoclamp. All other patients had uneventful outcomes and normal MV function at 3-month’s follow-up. The authors demonstrate in this study that re-operative MV surgery can be performed safely using a right mini-thoracotomy in patients with a previous sternotomy (30).
Similarly, Umakanthan and colleagues at Vanderbilt performed 90 consecutive minimally invasive MV operations in patients who had undergone previous cardiac surgery. 89% underwent MVR and 11% MVr through a right lateral thoracotomy approach with fibrillation. Cardiopulmonary bypass was instituted through axillary, femoral, or direct aortic cannulation. Operative mortality was 2%; lower than the STS-predicted mortality of 7%. Three patients developed acute renal failure postoperatively, one patient required hemodialysis, and one developed postoperative stroke. No patients developed postoperative myocardial infarction and the mean postoperative packed red blood cell transfusion requirement was two units. The authors concluded that MVr through a right thoracotomy with fibrillation is a safe and effective alternative to conventional redo-sternotomy for re-operative MV surgery (31).
From 1996 and 2010, Chitwood et al. performed re-operative minimally invasive MVr through a right mini-thoracotomy in 167 patients. Fibrillation was used in 77% and aortic clamping and root cardioplegia in 23%. Thirty-day mortality was 3.0% and from 2005-2010 that was decreased to no mortalities. There were no conversions to sternotomy or aortic dissections and stroke occurred in 2.4%. Overall they found that increased New York Heart Association functional class was the only independent predictor of mortality in this group of patients (32).
In a meta-analysis, the effects of minimally invasive MV surgery on morbidity and mortality were compared with conventional MV surgery. The results show equivalent peri-operative mortality, reduced need for reoperation for bleeding, and a trend towards shorter hospital stays. These benefits existed despite longer cardiopulmonary bypass and cross-clamp times in the minimally invasive group. Case-control studies from this meta-analysis showed consistently less pain and faster recovery as compared to traditional sternotomy. Data for minimally invasive MV surgery in the re-operative patient showed reduced blood loss, fewer transfusions, and faster recovery compared to re-operative sternotomy. Long-term follow-up data from multiple cohort studies revealed equivalent survival and freedom from re-operation (33).
Another meta-analysis using a comprehensive search of MEDLINE, Cochrane Library, EMBASE, CTSnet, and databases of abstracts identified 35 studies of minimally invasive MV surgery through a thoracotomy versus through median sternotomy. This meta-analysis reported that the mortality rate was similar between the two groups at 30 days, 1 year, 3 years, and 9 years. There was an improvement in the rates of atrial fibrillation, chest tube drainage, transfusions, sternal infection, time to return to activity, and patient scar satisfaction in the minimally invasive group. However, the 30-day risk of stroke, aortic dissection or injury, groin infection, and phrenic nerve injury were significantly increased for the minimally invasive group. Cross-clamp time, cardiopulmonary bypass time, and procedure time were significantly increased with the minimally invasive MV surgery but ventilation time and length of stay in the ICU and hospital were reduced (34).
Overall these studies and meta-analyses point to the safety, feasibility, and durability of MVr through a right mini-thoracotomy as compared to a traditional median sternotomy approach.
Conclusions
With over 35 years of institutional experience in mitral valve repair, we have demonstrated that a minimally invasive mitral valve repair performed through a right mini-thoracotomy is a safe and effective procedure, with early and late outcomes being equivalent to those achieved with traditional median sternotomy (13,21,24). Long term survival, freedom from recurrent MR, and freedom from reoperation are comparable and have not been compromised by this surgical approach (24). The right mini-thoracotomy approach provides the benefits of improved cosmesis, reduced post-operative pain, less blood loss with fewer blood transfusions, fewer infections, shorter length of stay, and faster return to activity (20). Based on our extensive experience we believe that mitral valve repair through a right mini-thoracotomy provides a durable and safe alternative to a traditional sternotomy; it is our standard of care approach for mitral valve surgery.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Carpentier A, Chauvaud S, Fabiani JN, et al. Reconstructive surgery of mitral valve incompetence: ten-year appraisal. J Thorac Cardiovasc Surg 1980;79:338-48. [PubMed]
- Carpentier A. Cardiac valve surgery--the “French correction”. J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed]
- Spencer FC, Colvin SB, Culliford AT, et al. Experiences with the Carpentier techniques of mitral valve reconstruction in 103 patients (1980-1985). J Thorac Cardiovasc Surg 1985;90:341-50. [PubMed]
- Galloway AC, Colvin SB, Baumann FG, et al. Long-term results of mitral valve reconstruction with Carpentier techniques in 148 patients with mitral insufficiency. Circulation 1988;78:I97-105. [PubMed]
- Galloway AC, Colvin SB, Baumann FG, et al. A comparison of mitral valve reconstruction with mitral valve replacement: intermediate-term results. Ann Thorac Surg 1989;47:655-62. [PubMed]
- Schwartz DS, Ribakove GH, Grossi EA, et al. Minimally invasive mitral valve replacement: port-access technique, feasibility, and myocardial functional preservation. J Thorac Cardiovasc Surg 1997;113:1022-30; discussion 1030-1. [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:567-74; discussion 574-6. [PubMed]
- Spencer FC, Galloway AC, Grossi EA, et al. Recent developments and evolving techniques of mitral valve reconstruction. Ann Thorac Surg 1998;65:307-13. [PubMed]
- Glower DD, Siegel LC, Frischmeyer KJ, et al. Predictors of outcome in a multicenter port-access valve registry. Ann Thorac Surg 2000;70:1054-9. [PubMed]
- Navia JL, Cosgrove DM 3rd. Minimally invasive mitral valve operations. Ann Thorac Surg 1996;62:1542-4. [PubMed]
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421-6; discussion 427-8. [PubMed]
- Grossi EA, Ribakove G, Schwartz DS, et al. Port-access approach for minimally invasive mitral valve surgery. Operative Techniques in Cardiac and Thoracic Surgery 1998;3:32-46.
- Colvin SB, Galloway AC, Ribakove G, et al. Port-Access mitral valve surgery: summary of results. J Card Surg 1998;13:286-9. [PubMed]
- Fann JI, Pompili MF, Stevens JH, et al. Port-access cardiac operations with cardioplegic arrest. Ann Thorac Surg 1997;63:S35-9. [PubMed]
- Grossi EA, Loulmet DF, Schwartz CF, et al. Evolution of operative techniques and perfusion strategies for minimally invasive mitral valve repair. J Thorac Cardiovasc Surg 2012;143:S68-70. [PubMed]
- Vernick WJ, Woo JY. Anesthetic considerations during minimally invasive mitral valve surgery. Semin Cardiothorac Vasc Anesth 2012;16:11-24. [PubMed]
- Aklog L, Adams DH, Couper GS, et al. Techniques and results of direct-access minimally invasive mitral valve surgery: a paradigm for the future. J Thorac Cardiovasc Surg 1998;116:705-15. [PubMed]
- Saunders PC, Grossi EA, Sharony R, et al. Minimally invasive technology for mitral valve surgery via left thoracotomy: experience with forty cases. J Thorac Cardiovasc Surg 2004;127:1026-31; discussion 1031-2. [PubMed]
- Repossini A, Kotelnikov IN, Parenzan L, et al. Left-side approach to the mitral valve. J Heart Valve Dis 2001;10:591-5. [PubMed]
- Grossi EA, Galloway AC, Ribakove GH, et al. Impact of minimally invasive valvular heart surgery: a case-control study. Ann Thorac Surg 2001;71:807-10. [PubMed]
- Grossi EA, LaPietra A, Ribakove GH, et al. Minimally invasive versus sternotomy approaches for mitral reconstruction: comparison of intermediate-term results. J Thorac Cardiovasc Surg 2001;121:708-13. [PubMed]
- Grossi EA, Galloway AC, LaPietra A, et al. Minimally invasive mitral valve surgery: a 6-year experience with 714 patients. Ann Thorac Surg 2002;74:660-3; discussion 663-4. [PubMed]
- Sharony R, Grossi EA, Saunders PC, et al. Minimally invasive reoperative isolated valve surgery: early and mid-term results. J Card Surg 2006;21:240-4. [PubMed]
- Galloway AC, Schwartz CF, Ribakove GH, et al. A decade of minimally invasive mitral repair: long-term outcomes. Ann Thorac Surg 2009;88:1180-4. [PubMed]
- Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg 2008;34:760-5. [PubMed]
- Holzhey DM, Shi W, Borger MA, et al. Minimally invasive versus sternotomy approach for mitral valve surgery in patients greater than 70 years old: a propensity-matched comparison. Ann Thorac Surg 2011;91:401-5. [PubMed]
- Gammie JS, Bartlett ST, Griffith BP. Small-incision mitral valve repair: safe, durable, and approaching perfection. Ann Surg 2009;250:409-15. [PubMed]
- D’Alfonso A, Capestro F, Zingaro C, et al. Ten years’ follow-up of single-surgeon minimally invasive reparative surgery for degenerative mitral valve disease. Innovations (Phila) 2012;7:270-3. [PubMed]
- Goldstone AB, Atluri P, Szeto WY, et al. Minimally invasive approach provides at least equivalent results for surgical correction of mitral regurgitation: a propensity-matched comparison. J Thorac Cardiovasc Surg 2013;145:748-56. [PubMed]
- Onnasch JF, Schneider F, Falk V, et al. Minimally invasive approach for redo mitral valve surgery: a true benefit for the patient. J Card Surg 2002;17:14-9. [PubMed]
- Umakanthan R, Petracek MR, Leacche M, et al. Minimally invasive right lateral thoracotomy without aortic cross-clamping: an attractive alternative to repeat sternotomy for reoperative mitral valve surgery. J Heart Valve Dis 2010;19:236-43. [PubMed]
- Arcidi JM Jr, Rodriguez E, Elbeery JR, et al. Fifteen-year experience with minimally invasive approach for reoperations involving the mitral valve. J Thorac Cardiovasc Surg 2012;143:1062-8. [PubMed]
- Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34:943-52. [PubMed]
- Cheng DC, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84-103. [PubMed]