Drugs pharmacokinetics during veno-venous extracorporeal membrane oxygenation in pediatrics
Pediatric pharmacokinetics (PKs): basic considerations
Pediatric drug therapy is considered a complex topic because children are considered a special population due to many physiological changes that take place during infancy and childhood. These changes have a tremendous impact on drugs’ PK and pharmacodynamic (PD).
Factors affecting site-specific drugs concentration over time include absorption, distribution, metabolism and excretion. These processes differ in pediatric population compared with adults altering the PK profile of a drug. Therefore, basic knowledge of these steps can be useful to ensure effective therapy in pediatric populations (1-5).
Absorption
Few PK data from clinical studies performed in the pediatric population limits the knowledge regarding the mechanisms of absorption, however due to the specific population evaluated in this context (critically ill pediatric patients undergoing extracorporeal membrane oxygenation), we will skip this part, because we will only evaluate intravenously administered drugs where absorption is considered complete (100%).
Distribution
Developmental changes in body composition affect the physiologic spaces where a drug can be distributed. The distribution of a drug can affect both its efficacy and duration (6). The distribution is dependent mainly on body composition that changes with age. Lipophilic drugs have a larger volume of distribution (Vd) in infants compared with toddler (>12–23 months) and preschool children (2–5 years) due to their higher levels of fat (7). Hydrophilic drugs also have a larger Vd in infants, toddler, and preschool children compared with adults. This because extravascular water decreases during development (8). As a consequence of higher volumes of distribution in infants, higher doses per kilogram body weight must be given to infants compared with adults to achieve comparable plasma and tissue concentrations (9,10). Changes in the composition and amount of circulating plasma proteins can also affect the distribution of highly protein-bound drugs. A reduction in the quantity of albumin in neonates and young infants increase the free fraction of drugs influencing the availability (11).
Metabolism
Liver enzyme and microsomal proteins increase progressively from an estimated 26 mg/g in newborns to a maximum of 40 mg/g in adult (12). Thus, drugs that are highly metabolized in the liver are given at lower mg/kg dose in neonates compared with toddler and preschool children. However, infants and toddlers may have a higher hepatic clearance (Cl) of drugs since hepatic blood flow is increased compared with adults, owing to the larger ratio of liver to total body mass in this population (13). This impacts dose adjustment and mg/kg dose scaling is often not adequate. Hepatic metabolism via cytochrome P450 (CYP) and uridine diphosphate glucuronosyltransferase (UGT) are markedly different in the neonatal period compared with the pediatric and adult population (14). The expression of phase I enzymes such as the P-450 cytochromes (CYPs) changes markedly during child development. Different patterns of isoform-specific expression of CYPs have been observed postnatally (15). Within hours after birth, CYP2E1 activity develops and CYP2D6 becomes detectable early after. CYP3A4 and CYP2C appear start their activity during the first week of life, whereas CYP1A2 system is the last hepatic CYP and starts working at 1 or 3 months of life (15-17). For instance, intravenously administered midazolam is cleared from the plasma by the CYP3A4 and CYP3A5 enzymes and the grade of this activity increases progressively from 1.2 to 9 mL per minute per kg of body weight during the first three months of life (18).
The ontogeny of conjugation reactions (isoforms of glucuronosyltransferase-UGT) instead is not well established like the one involving the phase I enzymes. The glucuronidation of morphine (19) can be detected in premature infants from 24 weeks of gestational age and its Cl can be positively correlated with the post-conceptional age. Morphine Cl quadruples between 24 and 40 weeks post-conceptual age, thereby necessitating a proportional increase in the dose of morphine to maintain an effective analgesia (20).
Renal elimination
Kidneys perform the elimination of many drugs and their metabolites. The glomerular filtration rate (GFR) is 2–4 mL/min/1.73 m2 in a full-term newborn and doubles after 1 week of age, reaching the adult levels at the end of the first year of life. The renal Cl of unchanged drug is generally lower in neonates owing to the immaturity of their renal excretion system. Anyway, a similar or a greater rate of renal Cl has been observed in infants and toddlers compared with adults. This is due to the fact that the kidneys are several-fold greater in preschool children compared with adults (21). Creatinine Cl is often used to estimate the GFR in children and a reduction in the drug dose is advised if creatinine Cl is less than the normal GFR. Urinary pH can also impact on the reabsorption of weak acid or basis which can influence the excretion of drugs and their metabolites. Infant pH is lower than adult values and this aspect may also increase the reabsorption of the weakly acidic drugs (22-24).
PK changes during extracorporeal membrane oxygenation (ECMO)
Neonatal and pediatric patients on ECMO are daily treated with many different drugs (1,25). These patients receive heparin to avoid clotting, sedatives and opioids for sedation, antibiotics, antivirals or antifungals to treat infections. ECMO can alter the drugs PK within two main mechanisms: drugs to circuit absorption; increase in Vd with or without changes in the drug Cl. The interaction between the drug and the ECMO circuit has been studied primarily in ex vivo experiments in which drug is administered to isolated ECMO circuits. Since no patient is connected to the circuit, any changes in the drug concentration over time is due to the circuit absorption and to the drug degradation (Table 1).
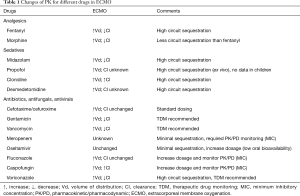
Full table
The whole circuit composed by polyvinylchloride (PVC) tubing and a membrane oxygenator, provides a large surface area not only for binding drugs but also for releasing drugs once the intermittent or continuous administration is stopped. Generally, the mechanism of sequestration to the circuit is due to the drugs’ chemical property, in particular to the drugs’ lipophilicity (26,27). The process of absorption is mediated by the electrostatic and hydrophobic interactions. The electrostatic interactions tend to dominate when the surface coatings are applied to the ECMO circuit components, while the hydrophobic interactions tend to dominate when hydrophobic drugs adhere to hydrophobic alkyl groups on polymers present on PVC tubing and membrane oxygenator without coating (1,27).
Surface coatings are increasingly applied to the ECMO circuit components to minimize the inflammatory response triggered when blood comes into contact with a foreign material (28,29), but coating also can change the nature of interaction between the surfaces of the circuit and the drugs. Absorption depends also on the number of binding sites on the surfaces and on the age of the circuits (older circuits seems to show higher losses), however conflicting data exist as to whether binding is saturable and about age-dependent saturation effect (30).
Ex vivo studies shows how the drugs sequestration into the ECMO circuit may alter their PK and PD. Drugs absorption has been tested for several drugs (31-34). The group of Wildschut et al. (25) demonstrated that the drug absorption is positively correlated with the grade of lipophilicity. Higher octanol/water partition coefficient (logP) values results in an increased drug loss (25). Compounds with a high logP are likely very soluble and may have high absorption in the ECMO circuit. Commonly used medication such as fentanyl, midazolam, propofol, dexmedetomidine and voriconazole have high logP value therefore dosages must be increased to reach the desired effect (31-34). Propofol, lorazepam and midazolam also showed a significant sequestration in ex vivo studies when using the newer oxygenators (polymethylpentene hollow-fibers) and PVC tubing (35-38). An ex vivo study of fentanyl (logP 4, protein binding 80%) in an ECMO circuit using a silicone membrane oxygenator showed >99% fentanyl loss in 180 min (25). The same experiments repeated in a circuit with a hollow-fiber polypropylene oxygenator showed a fentanyl loss of 66% at 180 min (25). Thus, lipophilic drugs will have a large Vd leading to lower circulating concentrations of drugs and potentially suboptimal pharmacologic therapy.
A second important aspect to explain the increase in Vd in pediatric patients is the hemodilution effect. Hemodilution has a tremendous impact on drugs whose distribution is limited to the plasma compartment (low Vd). Hemodilution effect is inversely related to age. In a neonate, the circuit priming can be 2–3 times the patient’s blood volume, whereas in a school age child (30 kg) it can be 20% of the child’s blood volume (39). All these physiologic features should take into account when prescribing drugs such as sedation and antibiotics, because doses could be increased to maintain adequate serum concentrations.
(Veno-venous ECMO) is generally performed to manage cases of respiratory failure due to infectious agents (bacterial, viral, etc.), both the infectious state and the use ECMO can contribute to the increase in the patient’s Vd. Both situations create an inflammatory state, inducing edema and often fluid overload, furthermore low saturation and hypoxia encountered before the beginning of VV-ECMO may contribute to reduce the regional kidney and hepatic blood affecting the drugs’ CI and metabolism. The association of renal replacement therapy in ECMO to manage fluid overload (1,2) may complicate this physiology while on ECMO complicating the PK changes.
Translating the results of ex vivo studies into clinical practice may be counter-productive. There is a large discordance between the drug absorption rates due to circuit sequestration in ex vivo tests and the reduced plasma concentration of drugs due to the increased Vd observed in the PK clinical studies in children on ECMO (1,2,40). Actually, we don’t know if this is due to the rapid distribution of the drug in the adipose tissues or if the continuous infusion rates are higher than absorption rates in ECMO circuit.
In conclusion, circuit sequestration impacts drugs availability. The increase in the Vd in pediatric patients is mainly due by the priming fluid composition and volume especially in infancy (1-3). The old silicone membrane oxygenator had a higher capacity of drug absorption compared to the newer ones in polymethylpentene. Most PK studies were performed with silicone oxygenators and tubing, thus the extrapolation of these data to the newer oxygenators and PVC tubing is not accurate. The ex vivo studies show a rapid drug absorption after the injection indicating that high lipophilic drugs should not administered directly into ECMO circuit. The relationship between logP values and the sequestration rate will support clinicians to predict the absorption of different drugs. These PK/PD considerations will help to understand why some drugs are more effective than other when administered in ECMO (e.g., morphine analgesia vs. fentanyl analgesia) or the necessity to administer a higher loading dose of sedatives or analgesics when initiating ECMO or the need to decrease the frequency of renally or hepatically excreted drugs if altered Cl is suspected (3). In any case, the therapeutic drug monitoring (TDM) is the best option to guide antibiotics dosing and other drugs with narrow therapeutic window. The association of the minimum inhibitory concentration (MIC) of antibiotics and the TDM will support clinicians to better control PK/PD effects while on ECMO.
Sedative and analgesic drugs
Actually, few data exist about sedation and analgesia in pediatric patients undergoing ECMO. Higher requirements of sedative drugs have been reported for these patients (41-44). Midazolam, fentanyl, and morphine are the drugs most commonly used and studied in the ECMO population.
Midazolam
Midazolam is a lipophilic drug (logP 3.9) with high protein binding (97%). Midazolam is metabolized in the liver by CYP3A4 and CYP3A5 to a hydroxylated metabolite (1-OH-midazolam) with subsequent metabolism to 1-OH-midazolam-glucuronide by UGT. Both metabolites are pharmacologically active when 1-OH-midazolam is nearly equipotent to the parent drug.
Actually, we have not any PK study on midazolam in older children supported with ECMO. PK studies in neonates and young infants on ECMO show a 3-fold to 4-fold increase in Vd after the initiation of ECMO for midazolam. This increase can be attributed mainly to the hemodilution and sequestration effect of midazolam by the ECMO circuit. Mulla et al. (45) described a reduced Cl for midazolam, resulting in accumulation of midazolam 48 hours after initiation of ECMO in term neonates. In the study by Ahsman et al. (40), midazolam Cl in term neonates increased overtime, possibly reflecting maturation of the CYP metabolizing enzymes and therefore the hepatic Cl. In contrast, renal Cl of the glucuronidation active metabolite of hydroxyl-midazolam was decreased (40).
Reduced Cl during ECMO greatly increases risk of adverse effects and efforts should be made to reduce midazolam infusions when possible based on clear treatment protocols.
Fentanyl
Fentanyl is a lipophilic drug (logP 4) with a high hepatic extraction ratio. Fentanyl is highly absorbed by the ECMO circuit (25). Koren et al. (46) reported high fentanyl sequestration in ECMO circuits with a subsequent need to increase fentanyl doses. High fentanyl infusion rates have been reported as also in other studies (41,47), indicating altered PK and PD, but conclusive PK results in neonates and children undergoing ECMO are still lacking.
The high adsorption rate of fentanyl in ECMO circuits may necessitate higher doses. Changes in the ECMO circuits with reduced capacity to adsorb drugs probably will reduce the fentanyl needs. A 48–72 hours rotation of opioids may reduce the chances to develop opioid withdrawal symptoms.
Morphine
Morphine has a low protein binding and is metabolized by the liver to active metabolites morphine-6-glucuronide by the enzyme UGT2B7 and morphine-3-glucuronide by UGT2B7 and the enzyme-family UGT1A.
Morphine is commonly used in neonatal intensive care both for analgesia and sedation. In 1994, Dagan et al. (43) showed a decrease in morphine Cl in neonatal ECMO runs, with a concomitant 2-fold increase after the ECMO withdrawal. Geiduschek et al. (48) found a similar change of the Cl of morphine in 11 neonates undergoing ECMO. Approximately half of the patients showed an increased Cl over time, probably due to the developmental drug maturation of the liver enzymes activity. However, Geiduschek et al. did not find any significant decrease of morphine levels directly after the ECMO withdrawal. These investigators concluded therefore that PK of morphine was not significantly altered during ECMO.
In 2006, Peters et al. reported a 2-fold increase of Vd for morphine in neonatal ECMO runs compared with postoperative patients not treated with ECMO. Furthermore, Cl was decreased at the start of ECMO but increased over time, with normal Cl for age at day 14. The Cl of morphine-3-glucoronide and morphine-6-glucoronide is related to creatinine Cl (49,50). The increased Vd found in ECMO patients is partly caused by dilution. Morphine sequestration is substantially less compared with more lipophilic drugs such as fentanyl and midazolam, explaining in part the reduced effect on Vd. Altered Cl may reflect severity of disease more than specific ECMO-related changes (51).
Propofol
Propofol is a highly protein-bound (95–99%) and highly lipophilic drug (logP 3.8). It is mainly metabolized in the liver by glucuronidation at the level of C1-hydroxyl end. Hydroxylation of the benzene ring to 4-hydroxy propofol may also occur via CYP2B6 and CYP2C9. In children, propofol is not used for long-term sedation because of the possible occurrence of propofol infusion syndrome (52,53).
There are no in vivo data on propofol during ECMO in pediatric population. In vitro studies show high adsorption rates by ECMO systems (54). This situation most likely results in increased Vd when used in ECMO patients.
In pediatric ECMO patients, higher doses of propofol bolus injections are probably caused by the increased Vd caused by hemodilution and drug adsorption to the ECMO circuit components. The concern for propofol infusion syndrome (55) make this drug less studied in pediatrics.
Dexmedetomidine and clonidine
Currently there are no published clinical studies of dexmedetomidine in ECMO. In an ex vivo study, Wagner et al. (36) reported that the loss of dexmedetomidine was due to the mechanism of circuit sequestration. Instead, as far as concerned clonidine, one clinical study was published by the group of Kleiber et al. (56) evaluated the use of clonidine for sedation in neonates and children undergoing VV-ECMO and continuous renal replacement therapy. They found an increased in VD and an increase in the Cl of the drug.
Antimicrobial drugs
Antibiotic use in ECMO patients is high, with a reported 71% use of antibiotic prophylaxis and 40% prolonged antibiotic use during ECMO (1). Infection rates in the pediatric ECMO population vary between 9% and 14% (57,58). Nosocomial or ongoing infections remain a significant problem and are associated with an increased ECMO mortality (58).
PK data on antibiotics on ECMO are limited, only vancomycin, gentamicin, and cefotaxime have been studied in detail. However, sequestration of antimicrobial drugs by the ECMO circuit is less pronounced compared with sedative drugs. Most antibiotics are excreted via the kidney. Addition of hemofiltration potentially increases Cl for drugs with a high unbound fraction.
Efficacy of some antibiotics leans on the peak concentration. This can be reduced when the Vd is increased. The risk of adverse events related to high levels may be increased because of the reduced Cl. For the antibiotics where the efficacy leans on the time greater than the minimum inhibitory concentration (MIC), PK/PD may be influenced by differences of both Cl and Vd.
Antibiotic dosing regimens in pediatric ECMO patients should take into account ECMO-related PK changes, different PK models should be applied especially when ECMO is associated with other extracorporeal therapy such as continuous renal replacement therapy which is often used in children to manage fluid overload.
Gentamicin
Gentamicin is an aminoglycoside antibiotic with high water solubility (logP −3) and low protein binding (0–30%). It is mainly eliminated unchanged via the kidneys.
Five studies examined gentamicin in infants on ECMO. All found an increased Vd, ranging from 0.51 (59) to 0.748 L/kg (60-63) compared with 0.45 L/kg in post-ECMO patients and critically non-ECMO patients. Gentamicin Cl was decreased while on ECMO compared with the post-ECMO period (62,63). It is unclear whether this decrease is because of ECMO itself or because of improvement in the clinical condition. When compared with septic full-term newborns, the Cl of gentamicin seems to be unaffected or slightly decreased (64). All studies showed an increased elimination half-life of 10 hours compared with 5–6 hours patients not receiving ECMO (64).
Different dosing regimens were used in the studies. None reflect the current dose recommendations.
The increased Vd reflects the findings in infants on ECMO and can mostly due to the dilution effect induced by the circuit priming and transfusions. The possible decrease in peak concentrations with prolonged elimination necessitates the use of TDM for these patients, especially during renal replacement therapy, in which serum creatinine does not accurately reflect renal function. The kinetically guided maintenance dosing of gentamicin based on plasma concentration after the first dose should be optimized despite high inter-individual PK parameter variability, especially in neonates treated for perinatal asphyxia with therapeutic hypothermia and multiorgan dysfunction syndrome (65).
Vancomycin
Vancomycin is a glycopeptide with a high-water solubility (logP −3.1) and moderate protein binding (55%). This drug exerts a high renal Cl by glomerular filtration.
Vd is either increased or unaffected by ECMO. Cl was decreased, but primarily correlated to renal function (31,66-68). A decrease in renal perfusion likely results in a decreased vancomycin Cl.
Thus, vancomycin pediatric dosing intervals should be based on age and renal function. TDM monitoring should be used to adjust dosing especially when vancomycin is used in continuous infusion.
Cephalosporins
Cephalosporins such as cefotaxime, cefuroxime, cefazolin are commonly used in children, especially when supported with ECMO. PK of cefotaxime and cefuroxime during ECMO is altered but plasma levels are more than the MIC using normal dosing regimens (69,70). Cefotaxime Cl was found to be increased during ECMO compared with the pre-ECMO and post-ECMO period, possibly reflecting improved hepatic or renal perfusion during ECMO or Cl via hemofiltration (69). Because of the large therapeutic window of cefotaxime, the chance of reaching toxic effects is small. Thus, both cefotaxime and cefazolin do not need dose adjustments in children supported with ECMO.
Meropenem
Pediatric data regarding the PK of meropenem in ECMO are limited to case reports. These small experiences do not show an increase in Vd but report an increase in Cl. Therefore, the recommended dose for meropenem (40 mg/kg bolus followed by a 200 mg/kg/die in continuous infusion) should be higher than the standard one (40 mg/kg every 8 hours) when the patient is on ECMO. The use of TDM coupled to MIC will allow clinicians to titrate the best dose for each patient (71-73).
Oseltamivir
During the H1N1 pandemia ECMO was successfully used both in children and adults with survival rates of 70% (74-76). Oseltamivir is commonly used to treat H1N1 influenza. A case report describing three patients undergoing ECMO for respiratory failure due to H1N1 showed that an adequate oseltamivir plasma level was achieved in two of three patients. In particular, a 2-fold dose increase of 4 mg/kg/die showed a parallel 2-fold increase in oseltamivir plasma level. It seems that the ECMO support does not impact oseltamivir levels in this report. Only one patient with decreased gastric motility did not achieved adequate plasma concentrations (77,78).
Fluconazole
Watt et al. (79) evaluated fluconazole with a single-center, open-label PK study and safety trial of prophylactic and treatment dosages (25 mg/kg once per week and 12 mg/kg/daily respectively) in infants receiving ECMO.
They found higher Vd and similar Cl of fluconazole for those on ECMO compared to historical control. Percentage of time above MIC was achieved for 78% of infants in prophylaxis while only 11% achieved the target plasma level required for treatment of invasive fungal infections. Minimal circuit sequestration of fluconazole was reported. Thus, TDM is recommended to manage severe invasive fungal infections.
Voriconazole
Due to its lipophilicity voriconazole has a high Vd, therefore a higher dose is recommended in children. These data come from case reports (14 mg/kg every 12 hours to achieve a target plasma concentration >1 mg/L), therefore TDM is recommended in severely ill children to achieve adequate plasma concentration (80,81).
Caspofungin
We have only one case report evaluating the use of caspofungin in pediatric ECMO. It seems that despite using a high dose of caspofungin, the patient never reached adequate plasma concentrations to manage the fungal infection because this drug reported high Cl while on ECMO. TDM is recommended when available (82).
Micafungin
A recent case series on micafungin in infants on ECMO showed 20–90% increase in Vd with high normal Cl. Based on the data collected in 12 infants the authors recommend a daily dose of 2.5 or 5 mg/kg/24 hours for prophylaxis of treatment respectively (83).
Conclusions
Defined and certain pediatric dose regimens are still lacking for many drugs especially in children undergoing ECMO. Our understanding of PK changes is still not adequate to establish a PK predictive model. The association of routine samplings associated to a PK analysis using the NONMEM methodology in combination with validated PD end points [the COMFORT behavioral scale (84) or face, legs, arms, cry, consolability (FLACC) scale (85)] will permit a better understanding of drug therapy in these special patients.
Overall, Vd changes occur rapidly at start of ECMO as a result of hemodilution and adsorption of drugs by the ECMO system. Initial changes in Vd may necessitate higher doses to achieve adequate plasma concentrations. If Vd is increased and stays unchanged, steady state levels are affected only by the dose rate and Cl rate.
In conclusion, the collaboration among researchers and the creation of a consortium among centers will allow to get more robust data in this special population. Multicentric open-label descriptive PK pediatric studies like the one performed in Australia and New Zealand by Shekar et al. (86) for adult patients on ECMO will be able to acquire data on drugs were TDM is not available at bedside.
Acknowledgements
None
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Wildschut ED, Ahsman MJ, Houmes RJ, et al. Pharmacotherapy in neonatal and pediatric extracorporeal membrane oxygenation (ECMO). Curr Drug Metab 2012;13:767-77. [Crossref] [PubMed]
- Wildschut ED, van Saet A, Pokorna P, et al. The impact of extracorporeal life support and hypothermia on drug disposition in critically ill infants and children. Pediatr Clin North Am 2012;59:1183-204. [Crossref] [PubMed]
- Himebauch AS, Kilbaugh TJ, Zuppa AF. Pharmacotherapy during pediatric extracorporeal membrane oxygenation: a review. Expert Opin Drug Metab Toxicol 2016;12:1133-42. [Crossref] [PubMed]
- Batchelor HK, Marriott JF. Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol 2015;79:395-404. [Crossref] [PubMed]
- Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med 2003;349:1157-67. [Crossref] [PubMed]
- Ginsberg G, Hattis D, Sonawane B, et al. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci 2002;66:185-200. [Crossref] [PubMed]
- Puig M. Body composition and growth. Nutrition in Pediatrics. 2nd ed. In: Walker WA, Watkins JB. editors. Hamilton, ON: BC Decke, 1996;122-41.
- Milsap RL, Jusko WJ. Pharmacokinetics in the infant. Environ Health Perspect 1994;102:107-10. [Crossref] [PubMed]
- Morselli P, Morselli R, Bossi L. Clinical pharmacokinetics in newborns and infants. Handbook of Clinical Pharmacokinetics. In: Gibaldi M, Prescott L. editors. New York: ADIS Health Sciences Press, 1983;98-141.
- Brown RD, Campoli-Richards DM. Antimicrobial therapy in neonates, infants and children. Clin Pharmacokinet 1989;17 Suppl 1:105-15. [Crossref] [PubMed]
- Ehrnebo M, Agurell S, Jalling B, et al. Age differences in drug binding by plasma proteins: studies on human foetuses, neonates and adults. Eur J Clin Pharmacol 1971;3:189-93. [Crossref] [PubMed]
- Barter ZE, Bayliss MK, Beaune PH, et al. Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: reaching a consensus on values of human microsomal protein and hepatocellularity per gram of liver. Curr Drug Metab 2007;8:33-45. [Crossref] [PubMed]
- Rane A. Drug disposition and action in infants and children. Pediatric Pharmacology Therapeutic Principles in Practice. In: Yaffe SJ, Aranda JV. editors. Philadelphia, PA: W B Saunders Co, 1992;10-21.
- Lacroix D, Sonnier M, Moncion A, et al. Expression of CYP3A in the human liver--evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem 1997;247:625-34. [Crossref] [PubMed]
- Vieira I, Sonnier M, Cresteil T. Developmental expression of CYP2E1 in the human liver. Hypermethylation control of gene expression during the neonatal period. Eur J Biochem 1996;238:476-83. [Crossref] [PubMed]
- Treluyer JM, Jacqz-Aigrain E, Alvarez F, et al. Expression of CYP2D6 in developing human liver. Eur J Biochem 1991;202:583-8. [Crossref] [PubMed]
- Sonnier M, Cresteil T. Delayed ontogenesis of CYP1A2 in the human liver. Eur J Biochem 1998;251:893-8. [Crossref] [PubMed]
- de Wildt SN, Kearns GL, Leeder JS, et al. Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet 1999;37:485-505. [Crossref] [PubMed]
- Miller RP, Roberts RJ, Fischer LJ. Acetaminophen elimination kinetics in neonates, children, and adults. Clin Pharmacol Ther 1976;19:284-94. [Crossref] [PubMed]
- Scott CS, Riggs KW, Ling EW, et al. Morphine pharmacokinetics and pain assessment in premature newborns. J Pediatr 1999;135:423-9. [Crossref] [PubMed]
- Anderson BJ, Holford NH. Understanding dosing: children are small adults, neonates are immature children. Arch Dis Child 2013;98:737-44. [Crossref] [PubMed]
- Chen N, Aleksa K, Woodland C, et al. Ontogeny of drug elimination by the human kidney. Pediatr Nephrol 2006;21:160-8. [Crossref] [PubMed]
- Schwartz GJ. Does kL/PCr estimate GFR, or does GFR determine k? Pediatr Nephrol 1992;6:512-5. [Crossref] [PubMed]
- Alcorn J, McNamara PJ. Using ontogeny information to build predictive models for drug elimination. Drug Discov Today 2008;13:507-12. [Crossref] [PubMed]
- Wildschut ED, Ahsman MJ, Allegaert K, et al. Determinants of drug absorption in different ECMO circuits. Intensive Care Med 2010;36:2109-16. [Crossref] [PubMed]
- Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet 2003;42:403-17. [Crossref] [PubMed]
- Shekar K, Roberts JA, Ghassabian S, et al. Altered antibiotic pharmacokinetics during extracorporeal membrane oxygenation: cause for concern? J Antimicrob Chemother 2013;68:726-7. [Crossref] [PubMed]
- De Somer F, François K, van Oeveren W, et al. Phosphorylcholine coating of extracorporeal circuits provides natural protection against blood activation by the material surface. Eur J Cardiothorac Surg 2000;18:602-6. [Crossref] [PubMed]
- Palatianos GM, Foroulis CN, Vassili MI, et al. A prospective, double-blind study on the efficacy of the bioline surface-heparinized extracorporeal perfusion circuit. Ann Thorac Surg 2003;76:129-35. [Crossref] [PubMed]
- Tayama E, Hayashida N, Akasu K, et al. Biocompatibility of heparin-coated extracorporeal bypass circuits: new heparin bonded bioline system. Artif Organs 2000;24:618-23. [Crossref] [PubMed]
- Mulla H, Pooboni S. Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation. Br J Clin Pharmacol 2005;60:265-75. [Crossref] [PubMed]
- Mehta NM, Halwick DR, Dodson BL, et al. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med 2007;33:1018-24. [Crossref] [PubMed]
- Mulla H, Lawson G, von Anrep C, et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion 2000;15:21-6. [Crossref] [PubMed]
- Dagan O, Klein J, Gruenwald C, et al. Preliminary studies of the effects of extra-corporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit 1993;15:263-6. [Crossref] [PubMed]
- Shekar K, Roberts JA, Mcdonald CI, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care 2012;16:R194. [Crossref] [PubMed]
- Wagner D, Pasko D, Phillips K, et al. In vitro clearance of dexmedetomidine in extracorporeal membrane oxygenation. Perfusion 2013;28:40-6. [Crossref] [PubMed]
- Lemaitre F, Hasni N, Leprince P, et al. Propofol, midazolam, vancomycin and cyclosporine therapeutic drug monitoring in extracorporeal membrane oxygenation circuits primed with whole human blood. Crit Care 2015;19:40. [Crossref] [PubMed]
- Harthan AA, Buckley KW, Heger ML, et al. Medication adsorption into contemporary extracorporeal membrane oxygenator circuits. J Pediatr Pharmacol Ther 2014;19:288-95. [PubMed]
- Sherwin J, Heath T, Watt K. Pharmacokinetics and Dosing of Anti-infective Drugs in Patients on Extracorporeal Membrane Oxygenation: A Review of the Current Literature. Clin Ther 2016;38:1976-94. [Crossref] [PubMed]
- Ahsman MJ, Hanekamp M, Wildschut ED, et al. Population pharmacokinetics of midazolam and its metabolites during venoarterial extracorporeal membrane oxygenation in neonates. Clin Pharmacokinet 2010;49:407-19. [Crossref] [PubMed]
- Leuschen MP, Willett LD, Hoie EB, et al. Plasma fentanyl levels in infants under-going extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 1993;105:885-91. [PubMed]
- Mulla H, Lawson G, Peek GJ, et al. Plasma concentrations of midazolam in neonates receiving extracorporeal membrane oxygenation. ASAIO J 2003;49:41-7. [Crossref] [PubMed]
- Dagan O, Klein J, Bohn D, et al. Effects of extracorporeal membrane oxygenation on morphine pharmacokinetics in infants. Crit Care Med 1994;22:1099-101. [Crossref] [PubMed]
- Arnold JH, Truog RD, Scavone JM, et al. Changes in the pharmacodynamic response to fentanyl in neonates during continuous infusion. J Pediatr 1991;119:639-43. [Crossref] [PubMed]
- Mulla H, McCormack P, Lawson G, et al. Pharmacokinetics of midazolam in neonates undergoing extracorporeal membrane oxygenation. Anesthesiology 2003;99:275-82. [Crossref] [PubMed]
- Koren G, Crean P, Klein J, et al. Sequestration of fentanyl by the cardiopulmo¬nary bypass (CPBP). Eur J Clin Pharmacol 1984;27:51-6. [Crossref] [PubMed]
- Arnold JH, Truog RD, Orav EJ, et al. Tolerance and dependence in neonates sedated with fentanyl during extracorporeal membrane oxygenation. Anesthesiology 1990;73:1136-40. [Crossref] [PubMed]
- Geiduschek JM, Lynn AM, Bratton SL, et al. Morphine pharmacokinetics during continuous infusion of morphine sulfate for infants receiving extracorporeal membrane oxygenation. Crit Care Med 1997;25:360-4. [Crossref] [PubMed]
- Peters JW, Anderson BJ, Simons SH, et al. Morphine pharmacokinetics during venoarterial extracorporeal membrane oxygenation in neonates. Intensive Care Med 2005;31:257-63. [Crossref] [PubMed]
- Peters JW, Anderson BJ, Simons SH, et al. Morphine metabolite pharmacokinetics during venoarterial extra corporeal membrane oxygenation in neonates. Clin Pharmacokinet 2006;45:705-14. [Crossref] [PubMed]
- Franck LS, Vilardi J, Durand D, et al. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. Am J Crit Care 1998;7:364-9. [PubMed]
- Cremer OL. The propofol infusion syndrome: more puzzling evidence on a complex and poorly characterized disorder. Crit Care 2009;13:1012. [Crossref] [PubMed]
- Okamoto MP, Kawaguchi DL, Amin AN. Evaluation of propofol infusion syndrome in pediatric intensive care. Am J Health Syst Pharm 2003;60:2007-14. [PubMed]
- Hynynen M, Hammaren E, Rosenberg PH. Propofol sequestration within the extracorporeal circuit. Can J Anaesth 1994;41:583-8. [Crossref] [PubMed]
- Diedrich DA, Brown DR. Propofol infusion syndrome in ICU. J Intensive Care Med 2011;26:59-72. [Crossref] [PubMed]
- Kleiber N, Mathôt RA, Ahsman MJ, et al. Population pharmacokinetics of intravenous clonidine for sedation during paediatric extracorporeal membrane oxygenation and continuous venovenous hemofiltration. Br J Clin Pharmacol 2017;83:1227-39. [Crossref] [PubMed]
- Brown KL, Ridout DA, Shaw M, et al. Healthcare-associated infection in pediatric patients on extracorporeal life support: the role of multidisciplinary surveil¬lance. Pediatr Crit Care Med 2006;7:546-50. [Crossref] [PubMed]
- Kaczala GW, Paulus SC, Al-Dajani N, et al. Bloodstream infections in pediatric ECLS: usefulness of daily blood culture monitoring and predictive value of biological markers. The British Columbia experience. Pediatr Surg Int 2009;25:169-73. [Crossref] [PubMed]
- Southgate WM, DiPiro JT, Robertson AF. Pharmacokinetics of gentamicin in neonates on extracorporeal membrane oxygenation. Antimicrob Agents Chemother 1989;33:817-9. [Crossref] [PubMed]
- Bhatt-Mehta V, Johnson CE, Schumacher RE. Gentamicin pharmacokinetics in term neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy 1992;12:28-32. [PubMed]
- Cohen P, Collart L, Prober CG, et al. Gentamicin pharmacokinetics in neonates undergoing extracorporal membrane oxygenation. Pediatr Infect Dis J 1990;9:562-6. [Crossref] [PubMed]
- Dodge WF, Jelliffe RW, Zwischenberger JB, et al. Population pharmacokinetic models: effect of explicit versus assumed constant serum concentration assay error patterns upon parameter values of gentamicin in infants on and off extra¬corporeal membrane oxygenation. Ther Drug Monit 1994;16:552-9. [Crossref] [PubMed]
- Munzenberger PJ, Massoud N. Pharmacokinetics of gentamicin in neonatal patients supported with extracorporeal membrane oxygenation. ASAIO Trans 1991;37:16-8. [Crossref] [PubMed]
- DiCenzo R, Forrest A, Slish JC, et al. A gentamicin pharmacokinetic population model and once-daily dosing algorithm for neonates. Pharmacotherapy 2003;23:585-91. [Crossref] [PubMed]
- Martínková J, Pokorná P, Záhora J, et al. Tolerability and outcomes of kinetically guided therapy with gentamicin in critically ill neonates during the first week of life: an open-label, prospective study. Clin Ther 2010;32:2400-14. [Crossref] [PubMed]
- Buck ML. Vancomycin pharmacokinetics in neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy 1998;18:1082-6. [PubMed]
- Amaker RD, DiPiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother 1996;40:1139-42. [PubMed]
- Hoie EB, Swigart SA, Leuschen MP, et al. Vancomycin pharmacokinetics in infants undergoing extracorporeal membrane oxygenation. Clin Pharm 1990;9:711-5. [PubMed]
- Ahsman MJ, Wildschut ED, Tibboel D, et al. Pharmacokinetics of cefotaxime and desacetylcefotaxime in infants during extracorporeal membrane oxygenation. Antimicrob Agents Chemother 2010;54:1734-41. [Crossref] [PubMed]
- Knoderer CA, Saft SA, Walker SG, et al. Cefuroxime pharmacokinetics in pediatric cardiovascular surgery patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2011;25:425-30. [Crossref] [PubMed]
- Cies JJ, Moore WS 2nd, Dickerman MJ, et al. Pharmacokinetics of continuous-infusion meropenem in a pediatric patient receiving extracorporeal life support. Pharmacotherapy 2014;34:e175-9. [Crossref] [PubMed]
- Di Nardo M, Cairoli S, Goffredo BM, et al. Therapeutic drug monitoring for meropenem after the extracorporeal membrane oxygenation circuit change in children: is it necessary? Minerva Anestesiol 2016;82:1018-9. [PubMed]
- Bradley JS, Sauberan JB, Ambrose PG, et al. Meropenem pharmacokinetics, pharmacodynamics, and Monte Carlo simulation in the neonate. Pediatr Infect Dis J 2008;27:794-99. [Crossref] [PubMed]
- Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 2009;302:1888-95. [Crossref] [PubMed]
- Buckley E, Sidebotham D, McGeorge A, et al. Extracorporeal membrane oxygenation for cardiorespiratory failure in four patients with pandemic H1N1 2009 influenza virus and secondary bacterial infection. Br J Anaesth 2010;104:326-9. [Crossref] [PubMed]
- Bessereau J, Chenaitia H, Michelet P, et al. Acute respiratory distress syndrome following 2009 H1N1 virus pandemic: when ECMO come to the patient bedside. Ann Fr Anesth Reanim 2010;29:165-6. [Crossref] [PubMed]
- Wildschut ED, de Hoog M, Ahsman MJ, et al. Plasma concentrations of oseltamivir and oseltamivir carboxylate in critically ill children on extracorporeal membrane oxygenation support. PLoS One 2010;5:e10938. [Crossref] [PubMed]
- Eyler RF, Klein KC, Mueller BA. The pharmacokinetics of oseltamivir and oseltamivir carboxylate in a critically ill pediatric patient receiving extracorporeal membrane oxygenation and continuous venovenous hemodialysis. J Pediatr Pharmacol Ther 2012;17:173-6. [Crossref] [PubMed]
- Watt KM, Gonzalez D, Benjamin DK Jr, et al. Fluconazole population pharmacokinetics and dosing for prevention and treatment of invasive Candidiasis in children supported with extracorporeal membrane oxygenation. Antimicrob Agents Chemother 2015;59:3935-43. [Crossref] [PubMed]
- Brüggemann RJ, Antonius T. Therapeutic drug monitoring of voriconazole in a child with invasive aspergillosis requiring extracorporeal membrane oxygenation. Ther Drug Monit 2008;30:643-6. [Crossref] [PubMed]
- Ruiz S, Papy E, Da Silva D, et al. Potential voriconazole and caspofungin sequestration during extracorporeal membrane oxygenation. Intensive Care Med 2009;35:183-4. [Crossref] [PubMed]
- Koch BC, Wildschut ED, Goede AL, et al. Insufficient serum caspofungin levels in a paediatric patient on ECMO. Med Mycol Case Rep 2012;2:23-4. [Crossref] [PubMed]
- Autmizguine J, Hornik CP, Benjamin DK Jr, et al. Pharmacokinetics and Safety of Micafungin in Infants Supported With Extracorporeal Membrane Oxygenation. Pediatr Infect Dis J 2016;35:1204-10. [Crossref] [PubMed]
- van Dijk M, de Boer JB, Koot HM, et al. The reliability and validity of the COMFORT scale as a postoperative pain instrument in 0 to 3-year-old infants. Pain 2000;84:367-77. [Crossref] [PubMed]
- Merkel SI, Voepel-Lewis T, Shayevitz JR, et al. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs 1997;23:293-7. [PubMed]
- Shekar K, Roberts JA, Welch S, et al. ASAP ECMO: Antibiotic, Sedative and Analgesic Pharmacokinetics during Extracorporeal Membrane Oxygenation: a multi-centre study to optimise drug therapy during ECMO. BMC Anesthesiol 2012;12:29. [Crossref] [PubMed]


