Anemia as a clinical marker of stable chronic obstructive pulmonary disease in the Korean obstructive lung disease cohort
Introduction
Chronic obstructive pulmonary disease (COPD) will be the 3rd leading cause of death in North America, and a national survey conducted in Korea showed the prevalence of COPD under the definition of the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) criteria was of 17.2% (men, 25.8%; women, 9.6%) for subjects older than 45 years (1,2).
Patients with COPD suffer from many concurrent comorbidities, some of which are deemed to be major causes of death in COPD (3). Anemia is one of the co-morbidities and is an independent risk factor for mortality in a subset of COPD patients (4,5). Correcting anemia can improve dyspnea by decreasing the work required for breathing and minute ventilation in COPD (6,7).
Previous studies have suggested that chronic inflammation is the main mechanism whereby anemia can develop in COPD, although the causes of anemia are thought to be multifactorial (8,9). Nevertheless, the mechanism of development for anemia and the impact of anemia on the prognosis of COPD remain poorly understood. Hence, this study was performed to search factors linked with anemia and to evaluate the role of anemia on the prognosis of COPD.
Methods
Study design
Patients & definition of each condition
We enrolled 407 COPD patients of the Korean obstructive lung disease (KOLD) prospective cohort who attended pulmonary clinics in 16 university affiliated hospitals on an outpatient basis from 3 provinces throughout Korea (246 patients from Seoul, 130 patients from Gyunggi-do, 31 patients from Gangwon-do) from June 2005 to January 2015 (Figure 1). Out of 957 patients who were referred for evaluation and management of airway disease before enrollment, 453 patients with asthma according to clinician’s discretion and 97 patients with non-smoking COPD were excluded from this study (Figure 1). Inclusion criteria of COPD were as follows: (I) age ≥40 years; (II) current or former smoker with a smoking history ≥10 pack-years; (III) post-bronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) <0.7; (IV) no or minimal abnormality on a chest radiography.
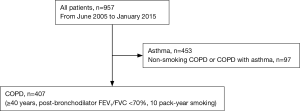
Patients with heart failure, coronary artery disease, tuberculous destroyed lung, severe bronchiectasis, pneumoconiosis, lung cancer, home oxygen therapy, or pure asthma were excluded based on the judgement of physicians.
For this cohort, we have followed the patients every 3 months and mean follow up period was 4.9±3.1 years.
Anemia was determined by initial serum hemoglobin level according to the reference values of hemoglobin: <12 g/dL for women and <13 g/dL for men (10).
Acute exacerbation was graded from mild to severe as previously reported during the 1-year follow up period after enrollment and only moderate and severe exacerbations were included in the exacerbation group (11). Frequent exacerbators were determined as previously defined (1,11).
This study was approved by the institutional review board of the Asan Medical Center (Approval No. 2005-0345) and the other 15 hospitals. Written informed consent was obtained from all patients.
Clinico-physiological indices of COPD
Demographic and clinical data, including age, sex, smoking status, history of exacerbations, pulmonary function tests, etc., were obtained at enrollment. Blood samples, collected at enrollment when the patients were stable, were used for laboratory tests. The body mass index (BMI) was calculated as the body weight divided by the height squared (kg/m2). Dyspnea was assessed using the Modified Medical Research Council (mMRC) Dyspnea Scale, and the health-related quality of life was assessed using the St. George Respiratory Questionnaire (SGRQ).
Six-minute walk test was conducted, according to the ATS statement (12).
Spirometry was performed using Vmax 22 (SensorMedics, Yorba Linda, CA, USA) and PFDX (MedGraphics, St. Paul, MN, USA) according to the American Thoracic Society guidelines (13). The spirometry reference values were based on the Korean equation (14). Post-bronchodilator spirometry values were measured 15 minutes after administering 400 µg of salbutamol. Lung volumes, including the total lung capacity (TLC) and residual volume (RV), were measured using body plethysmography which were V6200 (CareFusion, San Diego, CA, USA), PFDX, or Vmax 22 (15). The diffusing capacity of carbon monoxide (DLCO) was measured by assessing the single-breath carbon monoxide uptake (Vmax 22 or PFDX) (16).
Chest computed tomography (CT) indices for COPD assessment
The emphysema index, CT air-trapping index, and airway dimensions were determined from chest CT data, as previously reported (17,18). To measure these indices, patients underwent volumetric CT scans at full inspiration and expiration with a 16-MDCT scanner (the CT machines came from three manufacturers including the Somatom Sensation 16, Siemens Medical Solutions, Forchheim, Germany; the GE Lightspeed Ultra, General Electric Healthcare, Milwaukee, WI, USA; and the Philips Brilliance 16, Philips Medical Systems, Best, Netherlands).
Statistical analysis
All data were analyzed with SPSS 21.0 (SPSS Inc., Chicago, IL, USA), and all values were expressed as means ± standard deviation except for the survival period described as means ± standard error. Chi-Square test or Fisher’s exact test was performed for categorical data and Student’s t-test was used for continuous data. A P value less than 0.05 was considered statistically significant.
A Kaplan-Meier analysis with a log-rank test was conducted to evaluate the survival. A Cox proportional hazard regression analysis was carried out to find the significant variables associated with the survival. A Cox regression of the log hazard ratio (HR) on a covariate with a standard deviation of 0.50 based on a sample of 407 observations achieved 82% power at a 0.05 significance level.
Multiple logistic regression analyses were performed to choose the significant variables of the preceding analyses. The demographic variables, indicating the sample profile information including age and sex, and clinically meaningful variables are included in the multiple logistic regression. To avoid multicollinearity, the variables were included in multiple regression analyses when the variance inflation factor was less than 10.
Results
Baseline characteristics
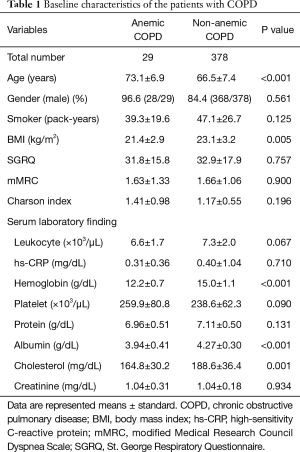
Our COPD cohort were male-dominant (male =97.3%, n=396), and 7.1% (n=29) of the entire group had anemia [mean cell volume (MCV) =93.6±5.0 fL, 96.6% (28/29) = normocytic anemia (MCV =80–100 fL), 3.4% (1/29) = macrocytic anemia (MCV ≥100 fL)]. The characteristics of the entire cohort included a mean age of 67.0±7.5 years, mean FEV1 of 49.1±15.5 (pre-bronchodilator) and 54.3%±16.0% (post-bronchodilator).
Compared to the non-anemic COPD group, the patients of the anemic COPD group were significantly older (P<0.001) and had lower BMI (P=0.005), lower serum albumin levels (P<0.001), and lower serum cholesterol levels (P=0.001) (Table 1). On the other hand, inflammatory markers such as the leukocyte count and high-sensitivity C-reactive protein (hs-CRP) level were not different between the two groups (Table 1).
Full table
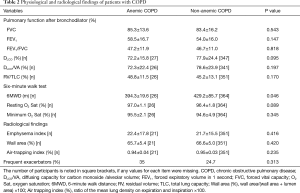
Radiological evaluations and pulmonary function tests including FVC, FEV1, FEV1/FVC, DLCO/VA (alveolar volume), DLCO, RV/TLC, and inspiratory capacity (IC)/TLC were not different between the two groups (Table 2). However, the 6-minute walk distance was shorter in the anemic COPD group (P=0.046) (Table 2).
Full table
Survival analyses
Of the 58 patients with COPD who died during the observation period, death was most frequently due to pneumonia (6 patients), respiratory failure (6 patients), acute exacerbation of COPD (6 patients), lung cancer (6 patients), cancers other than lung cancer (9 patients). Other causes were myocardial infarction (3 patients), respiratory failure after surgery (2 patients), septic shock (2 patients), pneumothorax (1 patient), liver cirrhosis (1 patient), multiorgan failure (1 patient) and suicide (1 patient). Since the cause of death was unidentified for 24.1% (14/58) of the instances, only all-cause mortality was used for the survival analyses.
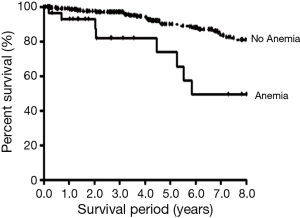
A Kaplan-Meier analysis showed that patients with non-anemic COPD (mean survival period =8.88±0.15 years) survived longer than those with anemic COPD (mean survival period =6.77±0.84 years, P<0.001) (Figure 2).
The multivariate analysis conducted with the Cox regression method including age, gender, mMRC, BMI, post-bronchodilator FEV1, 6-minute walk distance, and anemia revealed that age (HR =1.083, P=0.002), BMI (HR =0.840, P=0.001), post-bronchodilator FEV1 (HR =0.969, P=0.007), 6-minute walk distance (HR =0.995, P=0.008), and anemia (HR =2.591, P=0.025) were significant predictors of all-cause mortality (Table 3).
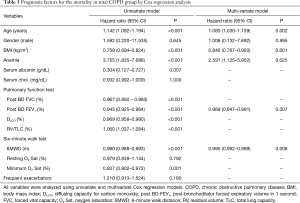
Full table
Factors associated with serum hemoglobin level
Multivariate logistic regression analysis using significant factors obtained from univariate analyses (Table 4) demonstrated that the independent factors associated with a lower serum hemoglobin level were a lower serum albumin level (t=5.902, P<0.001), older age (t=−4.087, P<0.001), male gender (t=3.326, P=0.001), and lower BMI (t=2.602, P=0.010), in the order of highest relation (Table 4). However, post-bronchodilator FEV1, DLCO, and the 6-minute walk distance were not independently associated (Table 4).
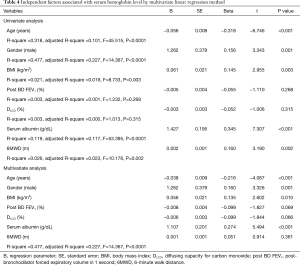
Full table
Discussion
The aim of our study was to investigate whether anemia can predict the survival of COPD and to search for factors that are independently associated with serum hemoglobin level in COPD. We found that anemia was a significant factor associated with the survival of COPD in the Cox regression analysis. Our data also demonstrated that nutritional factors, including a lower serum albumin level and lower BMI along with an increasing age and female gender were independent risk factors associated with a lower serum hemoglobin level in COPD, suggesting that aging and malnutrition are linked with anemia in COPD.
Our study has a number of interesting findings. First, our data showed that anemia was an independent factor to predict the survival of stable COPD in KOLD cohort. Previous studies with various cohorts reported that anemia is associated with a reduced survival in COPD (5,19-21). In the cohorts with stable COPD recruited on an outpatient basis, anemia has not been proven to be a risk factor for mortality through multivariate analysis, even though the survival period was reported to be shorter in anemic COPD, or hematocrit was significantly higher in the COPD patients who survived (19,20). However, in cohorts with severe COPD including COPD patients receiving long-term oxygen therapy and patients hospitalized for acute exacerbation, hematocrit or the presence of anemia was an independent predictor of survival (5,21).
As shown in the method, our cohort excluded COPD with cardiac comorbidities, tuberculous destroyed lung, severe bronchiectasis, pneumoconiosis, or lung cancer, because we attempted to observe the pure clinical course of COPD without the hindrance of those confounding variables.
It is no wonder that our cohort had a lower prevalence of anemia (7.1%) since patients with milder COPD and higher FEV1 were enrolled in KOLD cohort, compared to severe COPD cohorts as in previous research (mean FEV1 of 34% for men and 37% for women and an anemia rate of 12.6% for men and 8.2% for women reported by Chambellan et al., and mean FEV1 of 37.4% and anemia rate of 33% reported by Martinez-Rivera et al. (5,21).
A recent report by Ahn et al. identified serum hemoglobin levels and left ventricular ejection fraction along with a lower FEV1 as independent risk factors for mortality in COPD patients who underwent comprehensive cardiac evaluations (4). Although pulmonary function in the cohort reported by Ahn et al. (60.2%±18.7% of mean post-bronchodilator FEV1) was better than that of our cohort, their cohort is different from ours since their cohort recruited COPD patients with a higher prevalence of cardiac co-morbidities who simultaneously visited a cardiology clinic and a pulmonology clinic (4).
Previous reports indicated the possibility for the correction of anemia to improve the functional outcomes of COPD by decreasing dyspnea and the work during breathing (6,7). However, further research on COPD is required to determine the relationship between anemia correction and long-term outcomes.
Second, compared to non-anemic COPD, anemic COPD of the KOLD cohort had some distinct clinical features including a lower BMI, shorter 6-minute walking distance, lower albumin level and lower cholesterol level whereas inflammatory markers such as leukocyte count and hs-CRP did not exhibit differences between the two groups. A multivariate analysis showed that nutritional factors including a lower serum albumin level, lower BMI along with old age and gender were independent factors associated with the serum hemoglobin level in COPD, suggesting aging and malnutrition are linked with anemia in COPD.
It is not clear yet whether anemia in COPD is a part of the aging process independent of COPD progression or if it develops as a comorbidity of COPD.
Previous studies have reported that anemia mainly develops due to chronic inflammation in COPD because the low-grade systemic inflammation is associated with an increased risk of major co-morbidities in COPD, irrespective of smoking (9,22). However, multiple factors have been suggested to cause anemia in COPD in addition to systemic inflammation, including an increasing age, malnutrition, renal dysfunction, and the use of certain medicines such as theophylline and angiotensin-converting enzyme inhibitor (9,23).
Our data showed that inflammatory markers were not associated with the serum hemoglobin level, and this difference may be attributed to the fact that our cohort had milder COPD patients with a higher FEV1 enrolled from a stable cohort, when compared to previous studies (5,8).
The low body weight commonly found in COPD can weaken lung function and can reduce exercise capacity (24). Small studies identified dietary modulation as a promising intervention, and there is growing evidence that nutritional supplementation can improve body weight, respiratory muscle strength, and quality of life (24,25). However, nutritional intervention to improve the prognosis of COPD should be further validated in future studies.
We also acknowledge several limitations of this study. First, since the number of anemic patients was only 7.1% of our cohort, further validation of our observations seems to be necessary with a larger cohort. Second, our COPD cohort had male-dominant feature, since smoking is more prevalent in male in Korean population. Third, this study cannot determine a causal link between anemia and the associated factors. Fourth, the COPD assessment test score was not analyzed because it was not obtained at the initial enrollment. Fifth, the history of medication in our analysis was not taken into consideration because the current study was not a randomized controlled trial. Sixth, general population of Korea may not be represented by our subjects considering the feature of our men-dominant cohort and enrollment from three out of ten provinces of Korea.
Conclusions
In conclusion, our data suggested that in COPD, anemia was an independent risk factor for mortality, and nutritional factors such as a lower serum albumin level and lower BMI were independent factors associated with a lower hemoglobin level.
Acknowledgements
Funding: We thank the members of the KOLD Study Group for the provision of the KOLD cohort data built with the support of a grant from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (A102065).
Footnote
Conflicts of Interest: CK Rhee reports personal fees from MSD Korea, personal fees from AstraZeneca, personal fees from Novartis, personal fees from Mundipharma, personal fees from Boehringer-Ingelheim, outside the submitted work. YM Oh received payment for lecturing from MSD Korea, AstraZeneca Korea, Boehringer Ingelheim Korea, Novartis, DongWha, Takeda, and GSK Korea. SD Lee received support from Takeda Pharmaceuticals International GmbH for travel to an investigator meeting. He received payment from Takeda Pharmaceuticals International GmbH for attendance at an advisory board. He also received honoraria from GlaxoSmithKline, AstraZeneca, and Boehringer Ingelheim. The other authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of the Asan Medical Center (Approval No. 2005-0345) and the other 15 hospitals. Written informed consent was obtained from all patients.
References
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532-55. [Crossref] [PubMed]
- Kim DS, Kim YS, Jung KS, et al. Prevalence of chronic obstructive pulmonary disease in Korea: a population-based spirometry survey. Am J Respir Crit Care Med 2005;172:842-7. [Crossref] [PubMed]
- McGarvey LP, John M, Anderson JA, et al. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax 2007;62:411-5. [Crossref] [PubMed]
- Ahn YH, Lee KS, Park JH, et al. Independent risk factors for mortality in patients with chronic obstructive pulmonary disease who undergo comprehensive cardiac evaluations. Respiration 2015;90:199-205. [Crossref] [PubMed]
- Chambellan A, Chailleux E, Similowski T, et al. Prognostic value of the hematocrit in patients with severe COPD receiving long-term oxygen therapy. Chest 2005;128:1201-8. [Crossref] [PubMed]
- Schönhofer B, Wenzel M, Geibel M, et al. Blood transfusion and lung function in chronically anemic patients with severe chronic obstructive pulmonary disease. Crit Care Med 1998;26:1824-8. [Crossref] [PubMed]
- Silverberg DS, Mor R, Weu MT, et al. Anemia and iron deficiency in COPD patients: prevalence and the effects of correction of the anemia with erythropoiesis stimulating agents and intravenous iron. BMC Pulm Med 2014;14:24. [Crossref] [PubMed]
- John M, Hoernig S, Doehner W, et al. Anemia and inflammation in COPD. Chest 2005;127:825-9. [Crossref] [PubMed]
- Similowski T, Agusti A, MacNee W, et al. The potential impact of anaemia of chronic disease in COPD. Eur Respir J 2006;27:390-6. [Crossref] [PubMed]
- Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood 2006;107:1747-50. [Crossref] [PubMed]
- Oh YM, Sheen SS, Park JH, et al. Emphysematous phenotype is an independent predictor for frequent exacerbation of COPD. Int J Tuberc Lung Dis 2014;18:1407-14. [Crossref] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [Crossref] [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Hwang YI, Kim CH, Kang HR, et al. Comparison of the prevalence of chronic obstructive pulmonary disease diagnosed by lower limit of normal and fixed ratio criteria. J Korean Med Sci 2009;24:621-6. [Crossref] [PubMed]
- Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511-22. [Crossref] [PubMed]
- Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720-35. [Crossref] [PubMed]
- Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000;162:1102-8. [Crossref] [PubMed]
- Lee YK, Oh YM, Lee JH, et al. Quantitative assessment ofemphysema, air trapping, and airway thickening on computed tomography. Lung 2008;186:157-65. [Crossref] [PubMed]
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005-12. [Crossref] [PubMed]
- Cote C, Zilberberg MD, Mody SH, et al. Haemoglobin level and its clinical impact in a cohort of patients with COPD. Eur Respir J 2007;29:923-9. [Crossref] [PubMed]
- Martinez-Rivera C, Portillo K, Munoz-Ferrer A, et al. Anemia is a mortality predictor in hospitalized patients for COPD exacerbation. COPD 2012;9:243-50. [Crossref] [PubMed]
- Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:728-35. [Crossref] [PubMed]
- Yohannes AM, Ershler WB. Anemia in COPD: a systematic review of the prevalence, quality of life, and mortality. Respir Care 2011;56:644-52. [Crossref] [PubMed]
- Ferreira IM, Brooks D, White J, et al. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;12:CD000998. [PubMed]
- Schols AM. Nutrition as a metabolic modulator in COPD. Chest 2013;144:1340-5. [Crossref] [PubMed]


