Spontaneous breathing during veno-venous extracorporeal membrane oxygenation
Introduction
Veno-venous extracorporeal membrane oxygenation (VV ECMO) is going through a new era since the technological improvements over the last 10 years have made it safer and easier to use. Its clinical indications have gradually been extended to different pathologies [acute respiratory distress syndrome (ARDS), end-stage respiratory failure bridged to lung transplantation, chronic obstructive pulmonary disease (COPD)] and its use is widespread all over the world. As a direct consequence, this respiratory support has started to be considered as alternative to invasive ventilation, which is known to have serious adverse effects in many patients with respiratory failure (1,2). There may be several pathophysiological benefits of maintaining patients non intubated and spontaneously breathing: the best ventilation-perfusion matching would be preserved (3-5), the muscle tone would allow a better lung expansion at end expiration avoiding the formation of atelectasis (6-8), the incidence of ventilator/intubation-associated pneumonia would be reduced (9), the hemodynamic side effects of the sedative agents would decrease, the venous return would be enhanced by the lower intrathoracic pressure during spontaneous breathing and this mechanism could ameliorate cardiac filling and then cardiac output (10). Keeping patients awake can have some additional advantages: (I) the patients can actively perform physical rehabilitation leading to a reduction of the critically-ill polyneuropathy (11-13); (II) less use of sedatives could reduce the incidence of delirium (14,15); (III) moreover, the awake status allows the patient to communicate with nurse and medical staff and with relatives and friends.
However caution has been made using VV ECMO in awake spontaneous breathing conditions and one should not forget that you are handling a powerful and invasive device. The main warnings must be on the ventilation control: on the one hand unassisted spontaneous hyperventilation has to be avoided, since it could be as harmful as the invasive ventilation per se and could also mean higher work of breathing and increased oxygen consumption (16-19); on the other hand, hypoventilation (possible consequence of high levels of CO2 removal) has to be prevented, because it leads to derecruitment and worsening of the respiratory failure. Lastly, the risk of cannulae and devices displacement is higher in awake patients. For these reasons a close monitoring of awake patients on VV ECMO is desirable to minimize the possible drawbacks related to this approach.
In this article we will focus on the pathophysiology and the patient-machine interaction during awake ECMO, and we will also point out some of the technical aspects and monitor issues of the management of this invasive respiratory support in spontaneously breathing patients. The rationale for the awake VV ECMO in different types of respiratory failure will be discussed at the end of the paper.
Pathophysiology and patient-machine interactions
Extracorporeal gas exchange is driven by the same rules as in human physiology. Carbon dioxide removal is primarily dependent on ventilation (i.e., the fresh gas flow, being carbon dioxide content almost linearly related to carbon dioxide tension—Pco2) and to a lesser, but not ignorable extent on partial CO2 tension in the blood entering the circuit (input Pco2) and blood flow (in a logarithmic relationship) (20). On the other hand, oxygen transfer is greater with lower input saturation, but it is mainly dependent on perfusion (i.e., the extracorporeal blood flow), being blood oxygenation a saturable process due to the oxygen-hemoglobin association-dissociation curve (sigmoid shape) (21).
Practically, physicians change extracorporeal blood flow and gas flow to adjust oxygenation and decarboxylation, respectively. But the awake, spontaneously breathing patient is an independent and unpredictable variable interacting with ECMO support. This can occur both for mechanical reasons, with impact on ECMO drainage and re-infusion (i.e., extracorporeal blood flow), and for pathophysiological reasons, depending on patient response to ECMO CO2 clearance and O2 transfer.
Physio-metabolic interactions
In VV ECMO the total amount of extracorporeal blood flow is drained from and returned to the central venous compartment. Therefore, no direct hemodynamic modifications (e.g., change in preload or afterload) or heart support are generated, and all the effects on patient pathophysiology result from carbon dioxide removal and oxygen transfer by the artificial lung.
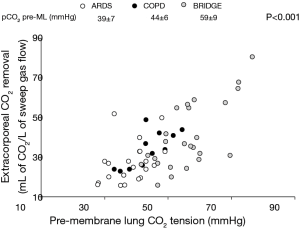
Carbon dioxide removal is the primary and more efficient effect of VV ECMO support. Correction of respiratory acidosis, if present, can reduce pulmonary vascular resistances, tachycardia and disproportionately high cardiac output (22). Some patients physiologically respond to CO2 removal with a proportional decrease in their alveolar ventilation, while some others do not (20,23,24). Reasons for not responding are various, including agitation and discomfort, cough, systemic consequences of the pulmonary disease (i.e., septic shock, hemodynamic derangement, fever, increased oxygen consumption), or different mechanisms involved in control of breathing and dyspnea maintenance other than pH/Pa
Non-responder patients will continue to hyperventilate despite CO2 removal. This augmented ventilation will lower Pa
Regarding oxygen transfer, correction of hypoxemia may per se reduce severe dyspnea in non-hypercapnic patients (28). By increasing venous oxygen saturation and venous oxygen tension VV ECMO could impair the physiologic response of hypoxic pulmonary vasoconstriction, thus increasing shunt (29). This could limit ECMO effect on arterial saturation, but could reduce pulmonary arterial pressures providing indirect right ventricular unloading (Figure 2).
Finally, the artificial lung is not only a gas exchanger, but a heat exchanger too. ECMO circuit acts as a “thermoregulating system” which modulates patient temperature and could often mask fever. This physical means of temperature control can be useful in sedated paralyzed septic patients, since reduction in body temperature will lower oxygen consumption and improve the V
Mechanical interactions
Extracorporeal blood flow depends on cannula type (diameter, holes disposition and the M-number) and position, and on central volume status (that is preload, venous return and central veins blood volume). Switch from positive pressure ventilation to spontaneous breathing can largely affect extracorporeal blood flow, as a consequence of the changes in the distribution of venous blood volume due to lung-heart interactions and of the preload dependency of centrifugal pumps.
Either an increase in extravascular pressure or a decrease in blood flow in the vein containing the drainage cannula, could cause vein collapse, leading to a reduction or even an interruption of blood flow. The hemodynamic effects of cyclic changes in intrathoracic pressure during spontaneous breathing are usually negligible in normovolemic patients with healthy lungs (30). Nevertheless, inspiratory intrathoracic pressure could become very negative and diaphragmatic excursion more pronounced in case of respiratory distress, causing both blood shift from inferior to superior vena cava and collapse of inferior vena cava due to increased abdominal pressure (31). The net result is a reduction in extracorporeal blood flow if the drainage cannula is positioned in the inferior vena cava (femoral cannulation). The effects of intrathoracic pressure on central blood volume also explains why the application of some kind of positive airway pressures (CPAP or NIV) can improve extrathoracic venous drainage (increased resistance to venous return causing increased blood volume in the inferior vena cava). A decrease in blood flow due to drainage difficulties can therefore induce a vicious circle in the awake patient. Any reduction in blood flow will result in reduction of extracorporeal oxygen delivery. If blood flow becomes very low (or even stops), CO2 removal will be impaired too, and patient will experience sudden dyspnea, which further compromises ECMO inflow, particularly in case of femoral cannulation. Temporary stop of centrifugal pumps (to interrupt the suctioning effect on vein walls), sedation (to reduce anxiety and inspiratory drive) and/or non-invasive ventilation (which supports dyspnea and increases intrathoracic pressure) may help interrupting this vicious circle. Bi-caval cannulas, draining from both intra- and extra-thoracic compartments, are less affected by this kind of interactions.
Monitoring during awake ECMO
Respiratory monitoring in awake, non-intubated patients has two major challenges: (I) Assessing the native lung function while respiratory gas exchanges are provided by the extracorporeal system; (II) evaluating respiratory pattern and work of breathing while patients are not connected to a ventilator.
Evaluation of native lung function
Oxygenation
VV ECMO cannot provide full arterial oxygenation, since it is a shunted circuit which can fully saturate a limited amount of cardiac output (no more than 50–60%). Moreover, oxygenation through the native lung may be further impaired by the partial reversal of hypoxic vasoconstriction induced by the increased PvO2 and by the imbalance of stoichiometric exchange of oxygen and carbon dioxide through the alveolar-capillary membrane induced by extracorporeal CO2 removal (as established by the alveolar gas equation with respiratory quotient as denominator). The latter effect states that native lung oxygenation properties cannot be properly evaluated without adequate CO2 exhalation. From a practical point of view, this means that oxygenation capability of native lung can be fully tested only when alveolar ventilation is restored reducing extracorporeal CO2 removal. With this known limitations, functional intrapulmonary shunt (calculated by means of central venous and arterial blood oxygen contents) is the best way to evaluate oxygen transfer by natural lungs.
Unfortunately, tidal volumes and therefore lung compliance cannot be measured in non-intubated patients.
Finally, regarding lung imaging, chest X-ray are feasible at the bedside, but radiologic improvement often follows by several days functional and clinical gain.
Decarboxylation
At steady state CO2 elimination equals CO2 production. During extracorporeal CO2 removal total CO2 elimination equals extracorporeal plus patient CO2 excretion. Extracorporeal CO2 clearance can be easily measured sampling the gas exiting the membrane lung (air gas-analysis) and multiplying the resulting CO2 content by the extracorporeal sweep gas flow. Conversely, quantification of natural lung CO2 excretion may be problematic in non-intubated patients, since no volumetric capnometry and/or tidal ventilation data are available. Side-stream capnography devices that measure end-tidal CO2 in spontaneously breathing patients are now available, but could give only an approximate idea of patient V
In conclusion, no absolute evaluation of both oxygenation performance and carbon dioxide elimination ability of the native lung can be done, until extracorporeal support is sustained. However, daily measurements of intrapulmonary shunt and artificial lung VCO2 under stable conditions allow an estimation of the relative contributions to respiratory gas exchange from the native and artificial lung. While absolute values of these two parameters have to be interpreted with caution, their temporal course allows to assess the evolution of native lung disease during the ECMO run.
Evaluation of patient’s respiratory pattern and efforts
With the possible rare exceptions of patients with tracheostomy, spirometric measures are not easily obtained and airways pressures and tidal volumes cannot be monitored. However, persistently high inspiratory efforts (i.e., high transpulmonary pressures) and/or high tidal volumes ventilation may worsen respiratory failure due to ventilation induced lung injury.
Clinical examination looking for signs and symptoms of respiratory distress (e.g., dyspnea, rapid shallow breathing pattern, use of accessory muscles of respiration, suprasternal and supraclavicular recession, active exhalation, etc.) and respiratory rate evaluation remain the keystones of respiratory monitoring in non-intubated, non-tracheostomized patients.
By providing a surrogate of pleural pressure measurement, esophageal pressure monitoring allows a bedside estimation of respiratory effort in these patients (33).
In ventilated patients esophageal trace shows positive excursions synchronous with mechanical inspiration, representing the amount of pressure spent for distending the thorax (i.e., it gives information about “chest wall” elastance). Subtracting this amount of pressure to the total driving pressure provided by the ventilator measured during an inspiratory pause (static conditions), we can compute the transpulmonary pressure, which is the driving pressure for lung inflation and the total stress applied to the lung. Conversely, esophageal trace shows an inspiratory decrease corresponding to the negative excursion of pleural pressure generated by the inspiratory muscles (i.e., the chest wall) and directly transmitted to the alveoli (so giving some information about the “lung”) during spontaneous unassisted inspiration. Differently from the situation above, the esophageal swings are not evaluated in static conditions, since no inspiratory/expiratory pauses can be done in non-intubated patients. Therefore, they represent the pressure applied to the alveoli to win both the elastic workload (i.e., transpulmonary pressure producing alveolar inflation) and the resistive workload (generating flow through the airways).
Both high transpulmonary pressures and high negative inspiratory pressures due to resistive workload are potentially injurious to the lung. In fact, negative pleural pressures against increased resistance decrease alveolar pressures without changing alveolar inflation (i.e., no stress and strain), but increase transmural capillary pressures (due to increased venous return, cardiac output, and left ventricular afterload) and could lead to hydrostatic pulmonary edema (ex-vacuo pulmonary edema). Moreover, it has been shown that this kind of pulmonary edema can induce (or worsen) lung inflammation (34).
In conclusion, the lack of tidal volumes and airway pressure monitoring remain a limitation in this approach, but our efforts are targeted to limit esophageal pressure swings, so as to ensure low transpulmonary pressures (which generate tidal volumes) and reducing the risk of worsening pulmonary edema in the setting of already increased lung permeability. Control of esophageal excursions is not different from control of dyspnea and patient’s respiratory drive, and can be achieved primarily manipulating fresh gas flow on ECMO, or with light sedation if this is not enough. When pleural swings and patient efforts are not controllable in this way, we believe it’s time to change to a more “conventional” invasive ventilatory strategy (i.e., intubation, deep sedation, and even paralysis).
Hemodynamics
Hemodynamic monitoring during awake ECMO is not different from what applied in the ventilated patients. In patients on ECMO support, we usually prefer invasive monitoring with the pulmonary artery catheter, which provides both cardiac output (thermodilution technique) and continuous direct pulmonary artery pressure measurements. Cardiac output is an important parameter to contextualize effective oxygenation support provided by ECMO, which is related to the extracorporeal blood flow to patient cardiac output ratio (providing that no significant recirculation occurs). Clinicians should be aware that subtraction artifacts may affect thermodilution measurements of cardiac output during ECMO support, with consequent overestimation errors, in particular when bi-caval cannulas or intrathoracic drainage are used. Pulmonary artery pressures measurements are of maximal relevance in case of right heart decompensation, but they also increased if lung derecruitment and collapse occur and could therefore be useful in non-intubated patients, in which classic respiratory mechanics (i.e., compliance) cannot be measured.
The rationale for awake VV ECMO in different types of respiratory failure
The awake ECMO approach has been consistently applied in patients with end-stage respiratory failure bridged to lung transplantation since less than 10 years ago (35-38). A randomized trial published by the Hannover group in 2012 showed a lower mortality in patients treated with the awake ECMO strategy compared with the conventional ECMO treatment in patients intubated on mechanical ventilation (36). The maintenance of the muscular tone with a daily physical activity seems to reduce the muscles deconditioning while waiting organ allocation. Moreover the avoidance of the intubation and mechanical ventilation reduce the incidence of respiratory and hemodynamic complications. The awake ECMO seems to reduce the postoperative complications after transplantation as well (11,38,39). Most of the transplant center around the world are now applying this strategy, as we do in our center. We documented that these patients consistently respond to the increase of the extracorporeal CO2 clearance reducing the respiratory rate and effort (24). Titration of extracorporeal support is essential to allow dyspnea relief while preventing the detrimental effects of hypoventilation, mainly derecruitment and inability to cough.
The rationale of the awake VV ECMO is different in COPD patients in whom the extracorporeal CO2 removal allows to reduce respiratory distress thus interrupting the vicious cycle of dynamic hyperinflation, which is often exacerbated by invasive mechanical ventilation. As COPD patients are often more hypercapnic than hypoxic, the hypoventilation-induced hypoxia is easily counterbalanced by increased inspired oxygen fraction through non-invasive devices. Moreover, clearance of 50–60% of the patient’s CO2 production could be enough to obtain relief from dyspnea in all COPD patients (24). These data can support the clinical use of less invasive low flow extracorporeal systems implemented in the last few years (40-44).
In our experience awake ECMO in ARDS patients is more complicated: respiratory drive control appears more difficult to achieve in this group and the risk of ventilation-induced lung injury is higher. The pathophysiology of ARDS, usually characterized by a high degree of lung inflammation, parenchymal consolidation and atelectasis that lead to low lung compliance, in addition to the concomitant extrapulmonary organ dysfunctions and often septic shock, could justify the lower or absent response to the extracorporeal CO2 removal. There are few experiences published on VV ECMO in ARDS so far (45,46). In our observational study (24) spontaneous breathing ECMO has been performed in only 27% of the ARDS patients and only 50% of them responded to the extracorporeal CO2 removal by decreasing significantly their respiratory rate and effort (Figure 3). Shunt fraction increased problematically and it was difficult to maintain oxygenation in a safe range.

Conclusions
Awake ECMO is a promising treatment strategy for patients with acute respiratory failure of different etiologies. This approach leads to a paradigm shift: while the conventional management of these patients with intubation and mechanical ventilation allows a close monitoring and a tight control of physiologic parameters, the awake spontaneously breathing patient is a less predictable and less controllable variable interacting with the artificial respiratory support. Safe and effective use of awake ECMO requires deep knowledge of patient cardiorespiratory pathophysiology and improved ability of monitoring its dynamic changes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. New Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Protti A, Cressoni M, Santini A, et al. Lung stress and strain during mechanical ventilation: any safe threshold? Am J Respir Crit Care Med 2011;183:1354-62. [Crossref] [PubMed]
- Putensen C, Mutz NJ, Putensen-Himmer G, et al. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1999;159:1241-8. [Crossref] [PubMed]
- Wrigge H, Zinserling J, Neumann P, et al. Spontaneous breathing improves lung aeration in oleic acid-induced lung injury. Anesthesiology 2003;99:376-84. [Crossref] [PubMed]
- Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 1974;41:242-55. [Crossref] [PubMed]
- Warner DO, Warner MA, Ritman EL. Human chest wall function while awake and during halothane anesthesia. I. Quiet breathing. Anesthesiology 1995;82:6-19. [Crossref] [PubMed]
- Hedenstierna G, Strandberg A, Brismar B, et al. Functional residual capacity, thoracoabdominal dimensions, and central blood volume during general anesthesia with muscle paralysis and mechanical ventilation. Anesthesiology 1985;62:247-54. [Crossref] [PubMed]
- Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med 2004;169:336-41. [Crossref] [PubMed]
- Hunter JD. Ventilator associated pneumonia. BMJ 2012;344:e3325. [Crossref] [PubMed]
- Funk DJ, Jacobsohn E, Kumar A. Role of the venous return in critical illness and shock: part II-shock and mechanical ventilation. Crit Care Med 2013;41:573-9. [Crossref] [PubMed]
- Rehder KJ, Turner DA, Hartwig MG, et al. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care 2013;58:1291-8. [Crossref] [PubMed]
- Latronico N, Fenzi F, Recupero D, et al. Critical illness myopathy and neuropathy. Lancet 1996;347:1579-82. [Crossref] [PubMed]
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874-82. [Crossref] [PubMed]
- Ouimet S, Kavanagh BP, Gottfried SB, et al. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med 2007;33:66-73. [Crossref] [PubMed]
- Hager DN, Dinglas VD, Subhas S, et al. Reducing deep sedation and delirium in acute lung injury patients: a quality improvement project. Crit Care Med 2013;41:1435-42. [Crossref] [PubMed]
- Hopkins SR, Schoene RB, Henderson WR, et al. Intense exercise impairs the integrity of the pulmonary blood-gas barrier in elite athletes. Am J Respir Crit Care Med 1997;155:1090-4. [Crossref] [PubMed]
- Mascheroni D, Kolobow T, Fumagalli R, et al. Acute respiratory failure following pharmacologically induced hyperventilation: an experimental animal study. Intensive Care Med 1988;15:8-14. [Crossref] [PubMed]
- Schmidt UH, Hess DR. Does spontaneous breathing produce harm in patients with the acute respiratory distress syndrome? Respir Care 2010;55:784-6. [PubMed]
- Yoshida T, Uchiyama A, Matsuura N, et al. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med 2012;40:1578-85. [Crossref] [PubMed]
- Kolobow T, Gattinoni L, Tomlinson TA, et al. Control of breathing using an extracorporeal membrane lung. Anesthesiology 1977;46:138-41. [Crossref] [PubMed]
- Spinelli E, Bartlett RH. Relationship between hemoglobin concentration and extracorporeal blood flow as determinants of oxygen delivery during venovenous extracorporeal membrane oxygenation: a mathematical model. ASAIO J 2014;60:688-93. [Crossref] [PubMed]
- Kiely DG, Cargill RI, Lipworth BJ. Effects of hypercapnia on hemodynamic, inotropic, lusitropic, and electrophysiologic indices in humans. Chest 1996;109:1215-21. [Crossref] [PubMed]
- Langer T, Vecchi V, Belenkiy SM, et al. Extracorporeal gas exchange and spontaneous breathing for the treatment of acute respiratory distress syndrome: an alternative to mechanical ventilation? Crit Care Med 2014;42:e211-20. [Crossref] [PubMed]
- Crotti S, Bottino N, Ruggeri GM, et al. Spontaneous breathing during extracorporeal membrane oxygenation in acute respiratory failure. Anesthesiology 2017;126:678-87. [Crossref] [PubMed]
- Scano G, Innocenti-Bruni G, Stendardi L. Do obstructive and restrictive lung diseases share common underlying mechanisms of breathlessness? Respir Med 2010;104:925-33. [Crossref] [PubMed]
- Gattinoni L, Iapichino G, Kolobow T. Hemodynamic, mechanical and renal effects during “apneic oxygenation” with extracorporeal carbon dioxide removal, at different levels of intrapulmonary pressure in lambs. Int J Artif Organs 1979;2:249-53. [PubMed]
- Field S, Kelly SM, Macklem PT. The oxygen cost of breathing in patients with cardiorespiratory disease. Am Rev Respir Dis 1982;126:9-13. [PubMed]
- Pesenti A, Rossi N, Calori A, et al. Effects of short-term oxygenation changes on acute lung injury patients undergoing pressure support ventilation. Chest 1993;103:1185-9. [Crossref] [PubMed]
- Benzing A, Mols G, Brieschal T, et al. Hypoxic pulmonary vasoconstriction in nonventilated lung areas contributes to differences in hemodynamic and gas exchange responses to inhalation of nitric oxide. Anesthesiology 1997;86:1254-61. [Crossref] [PubMed]
- Barnard M, Shukla A, Lovell T, et al. Esophageal-directed pressure support ventilation in normal volunteers. Chest 1999;115:482-9. [Crossref] [PubMed]
- Langer T, Santini A, Bottino N, et al. “Awake” extracorporeal membrane oxygenation (ECMO): pathophysiology, technical considerations, and clinical pioneering. Crit Care 2016;20:150-9. [Crossref] [PubMed]
- Monitoring VECO2 (carbon-dioxide elimination) during helmet-CPAP (Continuous Positive End-Expiratory Pressure): first experience. Available online: www.smartonweb.org
- Akoumianaki E, Maggiore SM, Valenza F, et al. PLUG Working Group. (Acute Respiratory Failure Section of the European Society of Intensive Care Medicine). The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014;189:520-31. [Crossref] [PubMed]
- Toumpanakis D, Kastis GA, Zacharatos P, et al. Inspiratory resistive breathing induces acute lung injury. Am J Respir Crit Care Med 2010;182:1129-36. [Crossref] [PubMed]
- Olsson KM, Simon A, Strueber M, et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant 2010;10:2173-8. [Crossref] [PubMed]
- Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8. [Crossref] [PubMed]
- Javidfar J, Brodie D, Iribarne A, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation and recovery. J Thorac Cardiovasc Surg 2012;144:716-21. [Crossref] [PubMed]
- Crotti S, Iotti GA, Lissoni A, et al. Organ allocation waiting time during extracorporeal bridge to lung transplant affects outcomes. Chest 2013;144:1018-25. [Crossref] [PubMed]
- Hayes D Jr, Kukreja J, Tobias JD, et al. Ambulatory venovenous extracorporeal respiratory support as a bridge for cystic fibrosis patients to emergent lung transplantation. J Cyst Fibros 2012;11:40-5. [Crossref] [PubMed]
- Burki NK, Mani RK, Herth FJ, et al. A novel extracorporeal CO(2) removal system: results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest 2013;143:678-86. [Crossref] [PubMed]
- Abrams DC, Brenner K, Burkart KM, et al. Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc 2013;10:307-14. [Crossref] [PubMed]
- Roncon-Albuquerque R Jr, Carona G, Neves A, et al. Venovenous extracorporeal CO2 removal for early extubation in COPD exacerbations requiring invasive mechanical ventilation. Intensive Care Med 2014;40:1969-70. [Crossref] [PubMed]
- Del Sorbo L, Pisani L, Filippini C, et al. Extracorporeal CO2 Removal in Hypercapnic Patients At Risk of Noninvasive Ventilation Failure: A Matched Cohort Study With Historical Control. Crit Care Med 2015;43:120-7. [Crossref] [PubMed]
- Beloncle F, Brochard F. Extracorporeal CO2 Removal for Chronic Obstructive Pulmonary Disease: Too Risky or Ready for a Trial? Crit Care Med 2015;43:245-6. [Crossref] [PubMed]
- Wiesner O, Hadem J, Sommer W, et al. Extracorporeal membrane oxygenation in a nonintubated patient with acute respiratory distress syndrome. Eur Respir J 2012;40:1296-8. [Crossref] [PubMed]
- Hoeper MM, Wiesner O, Hadem J, et al. Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in patients with acute respiratory distress syndrome. Intensive Care Med 2013;39:2056-7. [Crossref] [PubMed]


