Modified exposure method for gastric mobilization in robot-assisted esophagectomy
Introduction
During the last ten years, thoracolaparoscopy has been increasingly applied as a method of minimally-invasive esophagectomy to reduce morbidity and mortality. Nevertheless, some limitations have been met when performing minimally-invasive esophagectomy (1-3). For instance, rigid instruments decrease the amplitudes of maneuvers when operating in the posterior mediastinum, especially if the esophagus deviates to the left. Robotic surgical systems potentially provide a way to overcome these limitations by offering the use of 3-dimensional cameras and wristed instruments (1,4). However, stomach mobilization is usually a technically challenging procedure in the early stages of the learning curve associated with robot-assisted esophagectomy (5). As stomach retraction is hard to perform using robotic instruments (which lack tactile feedback), the conventional exposure method which employs a grasper fails to provide an adequate operative field. Thus, it has the potential to hurt the stomach and its important blood vessels (6). As a matter of fact, some surgeons prefer to perform a hybrid esophagectomy procedure, using a robotic system in the thoracic phase and laparoscopy or laparotomy in the abdominal phase (7-9).
When dissecting the gastrocolic ligament, it is crucial to keep the right gastroepiploic arcade adequately exposed in order to preserve it. The incidence of ischemia and necrosis of the gastric conduit has been extensively reported in minimally-invasive esophagectomy due to technical factors (10). Usually, dissection of the gastrocolic ligament is performed by traction of the stomach with a grasper during laparoscopic gastric mobilization. However, this may not be an easy task to accomplish in a robot-assisted procedure. Lack of tactile feedback and excessive force may lead to trauma caused by the handling of the stomach with the grasper (11). Moreover, numerous instruments inevitably interfere with each other. Similarly, the short gastric vessels are also difficult to expose because of the complexity of its anatomical location (12). To overcome these problems, we have developed a modified gastric mobilization technique which focuses on exposure methods.
Methods
Patients and analysis
A total of 59 consecutive patients, who underwent robot-assisted McKeown esophagectomy for esophageal squamous cancer at the West China Hospital from April 1st to December 31st, 2016, were enrolled in our prospective cohort. Preoperative diagnosis was based on gastroscopy and concomitant biopsy results. Tumors were evaluated to be resectable according to contrast-computed tomographic images of the chest and abdomen. The study protocol was approved by the appropriate institutional review board (number: 2017-239). Written informed consent was obtained from all patients. Descriptive statistics were used to describe the patients’ baseline. Univariate analysis was performed using nonparametric and chi-squared tests (SPSS 22; SPSS Inc., Chicago, IL, USA).
According to the technique for gastric retraction, we classified all patients into two groups: a grasper retraction (GR) group (n=27) and thread retraction (TR) group (n=32). To minimize the effects of the learning curve, the surgeon undertook months of training and worked with the robotic system in an esophagectomy context for three months before the study began. Moreover, we employed a two-step method to carry out GR/TR selection. First, we selected one of the two methods randomly. Then, after sufficient mutual preoperative communication with the patient about the surgical plan had taken place, the patient was given the right to decide which technique would be performed. The ‘gastric mobilization time’ was defined as the time interval between creating the first abdominal port to finishing the gastric mobilization. Postoperative morbidity events (minor or major) were graded according to CTCAT v4.0.
Surgical techniques
McKeown esophagectomy was performed in three phases using the da Vinci surgical system (Intuitive Surgical, Inc., Sunnyvale, CA, USA), that is to say, thoracic, abdominal, and cervical phases (13). Gastric mobilization is performed following the thoracic phase. First, the retraction of the liver, mobilization of the gastroesophageal junction, and dissection of the lesser curvature were performed as usual. Then, the left gastric artery and vein were transected with concomitant resection of local lymph nodes. Secondly, the right gastroepiploic arcade was exposed by retracting the stomach using a polyester tape combined with a thread loop. Finally, the mobilization was finished by dividing the gastrocolic ligament and transecting the short gastric vessels.
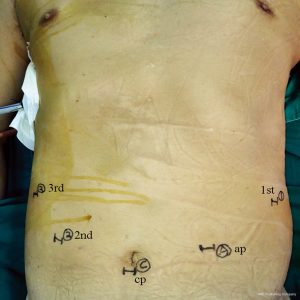
Five abdominal ports were first created in an identical way in all patients (Figure 1). A 12-mm viewing port was placed just below the umbilicus. Two 8-mm ports for robot-arms 1 and 3 were used below the left and right subcostal space, respectively. Another 8-mm port for arm 2 was placed at the umbilicus level in the right midclavicular line. Finally, a 12-mm accessory port was placed at the umbilicus level of the left midclavicular line. The robotic cart resides over the patient’s head. After retracting the left lobe of the liver and dissecting the lesser curvature, the left gastric vessels could be well exposed by grasping and lifting upwards the lesser curvature of the stomach using Cadiere Forceps (Intuitive Surgical, Inc., Sunnyvale, CA, USA) on arm 3. The abdominal lymph nodes around the left gastric artery were dissected en bloc using Fenestrated bipolar forceps (Intuitive Surgical, Inc.) on arm 2 and Harmonic scalpel (Ethicon, San Angelo, TX, USA) on arm 1. The left gastric vessels were then clipped using Hem-o-loks by an assistant surgeon and divided with the Harmonic scalpel. Division of the pancreaticogastric attachments was then performed using the Harmonic scalpel, leftwards to the splenic hilum.
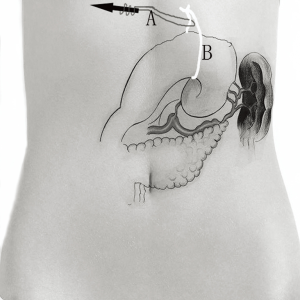
The above mentioned method of retracting the left lobe of the liver needs to be described further. As shown in Figure 2, the liver’s left lobe was lifted up using 2 slings of Prolene suture. First, a 70 mm straight needle with a polypropylene monofilament was introduced into the abdomen (4 cm below the xiphoid process, and slightly to the right). It then passed through the abdominal wall to the right of the round ligament of the liver. After the pars placcida of the hepatogastric ligament was divided, an intraperitoneal part of the Prolene was fixed at two ends of the pars condensa with Hem-o-lok clips. The Prolene was then tied outside of the abdomen (4 cm below xiphoid process, and slightly to the left) so that the left lobe of the liver was left hanging by the two slings.
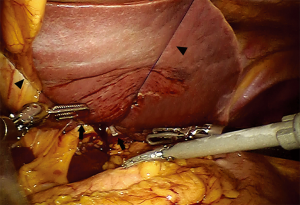
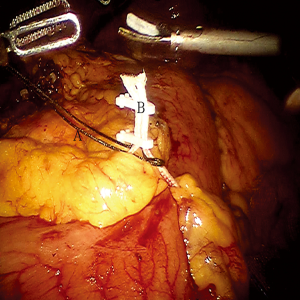
To obtain a satisfactory operative exposure and prevent stomach injury, gastric retraction was performed using polyester surgical tape. To begin with, a 2–3 cm hole was made in the great omentum. One end of the polyester tape was passed behind the stomach toward the lesser sac. Then, a thread loop was passed into the abdomen cavity in the subxiphoid region using a suture passer. One end of the polyester tape was passed through the loop, and then tied to the other end of the tape using a Hem-o-lok (Figures 3,4). In this way, the loop of polyester tape around the stomach was linked to the thread loop, and so the stomach could be lifted up by retracting the thread outside the abdomen. The subsequent gastric retraction allows for easy identification and preservation of the right gastroepiploic arcade (Figure 5) and short gastric vessels (Figure 6). Also, the greater curvature of the stomach is mobilized by dividing the gastrocolic omentum, followed by division of the short gastric vessels.
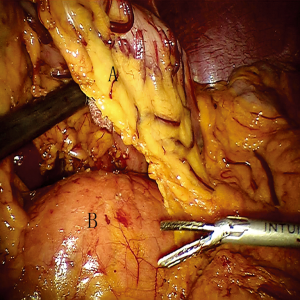
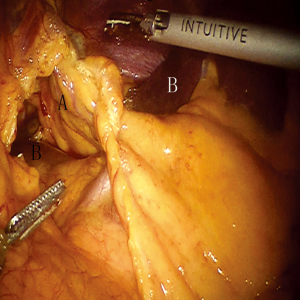
After gastric mobilization had finished, all subsequent manipulations were performed without using the robotic system. The stomach was pulled out of the abdominal cavity to create a gastric conduit with upper midline abdominal incisions. A (5 cm wide) gastric conduit was made, as is usual. Then, we sent back the gastric conduit to the abdominal cavity and then delivered it from the abdomen into the chest. Furthermore, an incision was made at the cervical level. The upper end of the gastric conduit in the thorax was delivered to the left neck. A purse-string was handsewn through the muscular layer of the esophagus 5–8 cm proximal to the esophageal cancer with one piece of 3-0 Prolene suture at the cervical level.
Results
The demographic and preoperative data are summarized in Table 1. A total of 59 patients (27 in GR group; 32 in TR group), including 44 male and 15 female, with a mean age of 61±9 years (range, 42–78 years) were enrolled in this prospective cohort. There were no cases converted to laparotomies. There was no incidence of postoperative 30-day mortality, and no obvious adverse injuries of the stomach were noticed.

Full table
The surgical outcomes are shown in Table 2. The median gastric mobilization time was 53 min (range, 38–77 min). Significantly less time was used in the TR group compared to the GR group (P=0.005), as shown in Figure 7. The median amount of blood loss during gastric mobilization was 8 mL (range, 5–14 mL), and no significant difference was found between the two groups (P=0.573). The median number of dissected lymph nodes was 10 (range, 7–16), and there was no significant between-group difference (P=0.386). Of the total 29 postoperative morbidity events, 16 occurred in 10 patients in the GR group, whereas 13 occurred in 9 patients in the TR group. These included anastomotic leakage, pneumonia, wound infection, and hoarseness. The postoperative morbidity rates did not differ statistically between the two groups (P=0.942).
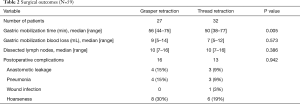
Full table
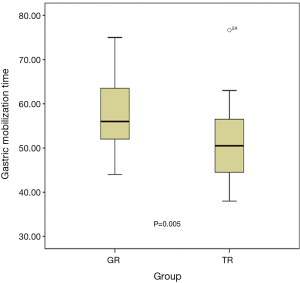
Discussion
The prospective comparative analysis shows that TR may be used to achieve a satisfactory operating space and shorten the operation time for patients undergoing robotic esophagectomy. The modified approach for overhanging the stomach makes it easier to mobilize the stomach and preserve important gastric vessels.
Even through robotic surgery is becoming increasingly more popular for performing esophagectomy, some limitations should be noticed. Most notably, it has been reported that the abdominal phase is not well suited to a robotic approach because large-amplitude maneuvers are required for dissection of the entire greater curvature (2). To achieve optimal surgical exposure and avoid injury to the right gastroepiploic arcade, the present study describes a modified procedure for gastric mobilization for use in robot-assisted esophagectomy. This paper appears to be the first report to focus on the exposure method in this respect.
Preservation of the right gastroepiploic arcade is crucial when dividing the gastrocolic ligament during gastric mobilization (14). Gastric tube complications, e.g. ischemia and necrosis of the gastric tube, are not rare after esophagectomy, the main technical factor being the arterial supply (10,15). During laparoscopic gastric mobilization, exposure of the right gastroepiploic vessels is usually achieved via two manipulations. One, is the lifting up of the stomach by grasping its anterior wall with atraumatic forceps; the other, is inserting a retractor between the stomach and pancreas and then holding the stomach up. In our experience, both manipulations are difficult to perform during robotic gastric mobilization. The lack of sensory feedback may increase the risk of potential organ injury (16). It is not uncommon for omental vessels to slip into the gaps between the robotic wrists and be torn when using the articulating instrument to elevate the stomach.
Another technical difficulty associated with gastric mobilization is to achieve a good exposure of the gastrosplenic ligament and short gastric vessels. Running through the gastrosplenic ligament are small branches from the short gastric and left gastroomental vessels. Due to their complex anatomical locations, intraoperative hemorrhage and spleen injury occasionally occur during robotic gastric mobilization (12,17). In the modified method presented, after using the thread loop to lift the stomach, the gastrosplenic ligament and short gastric vessels can be clearly visualized and easily dissected using a Harmonic scalpel.
In our opinion, most surgeons tend to slow down during gastric mobilization with robotic instruments to avoid unnecessary trauma to the arteries and organs. This may help explain the non-significant difference in leak rate and other complications between the two groups. However, we believe, that for those new to robotic esophagectomy, TR may be a more valuable procedure to reduce the risk of unexpected trauma.
We also calculated the sample size necessary for the observed leak difference to be statistically significant based on the present study. We found that, in total, more than one thousand cases would be required. Notably, this result may be strongly related to the surgeon’s experience.
Stomach mobilization is usually a technically challenging procedure in the early stages of the learning curve for robot-assisted esophagectomy. To the best of our knowledge, there nothing available in the literature that deals with this issue, so far. In fact, some surgeons prefer to perform a hybrid esophagectomy procedure, using robotic systems in the thoracic phase and laparoscopy or laparotomy in abdominal phase (7-9). In the technique presented here, we use polyester tape to retract the stomach atraumatically, which makes the omentum hang down curtain-like due to gravity. This method fulfills the requirement for adequate exposure of the right gastroepiploic arcade and short gastric vessels. It also provides a wide operative space far from the pancreas, spleen, and colon.
In conclusion, we have developed a modified exposure method for gastric mobilization for use in robot-assisted esophagectomy. The method may play an important role in preserving the right gastroepiploic arcade and short gastric vessels. Based on our initial experience with 59 patients, the method appears to be safe, easy, inexpensive, and effective.
Acknowledgements
Funding: This study was supported by grants from Chengdu City Science and Technology Project of China (No. 0040205301E42).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the appropriate institutional review board (number: 2017-239). Written informed consent was obtained from all patients.
References
- Osugi H, Takemura M, Lee S, et al. Thoracoscopic esophagectomy for intrathoracic esophageal cancer. Ann Thorac Cardiovasc Surg 2005;11:221-7. [PubMed]
- van Hillegersberg R, Boone J, Draaisma WA, et al. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc 2006;20:1435-9. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Finley DJ, et al. Combined thoracoscopic and laparoscopic robotic-assisted minimally invasive esophagectomy using a four-arm platform: experience, technique and cautions during early procedure development. Eur J Cardiothorac Surg 2013;43:e107-15. [Crossref] [PubMed]
- Boone J, Borel Rinkes IH, van Hillegersberg R. Robot-assisted thoracolaparoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc 2007;21:2342-3. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg 2013;145:90-6. [Crossref] [PubMed]
- Suda K, Nakauchi M, Inaba K, et al. Robotic surgery for upper gastrointestinal cancer: Current status and future perspectives. Dig Endosc 2016;28:701-13. [Crossref] [PubMed]
- Dapri G, Himpens J, Cadière GB. Robot-assisted thoracoscopic esophagectomy with the patient in the prone position. J Laparoendosc Adv Surg Tech A 2006;16:278-85. [Crossref] [PubMed]
- Kim DJ, Hyung WJ, Lee CY, et al. Thoracoscopic esophagectomy for esophageal cancer: feasibility and safety of robotic assistance in the prone position. J Thorac Cardiovasc Surg 2010;139:53-9.e1. [Crossref] [PubMed]
- Gronnier C, Piessen G, Mariette C. Laparoscopic gastric mobilization and lymphadenectomy during Ivor Lewis esophagectomy. J Visc Surg 2016;153:203-8. [Crossref] [PubMed]
- Ramage L, Deguara J, Davies A, et al. Gastric tube necrosis following minimally invasive oesophagectomy is a learning curve issue. Ann R Coll Surg Engl 2013;95:329-34. [Crossref] [PubMed]
- McGuire AL, Gilbert S. Transthoracic Extracorporeal Gastric Conduit Preparation for Minimally Invasive Ivor-Lewis Esophagectomy. Innovations (Phila) 2015;10:236-40; discussion 240. [Crossref] [PubMed]
- Ostermann PA, Schreiber HW, Lierse W. The ligament system of the spleen and its significance for surgical interventions. Langenbecks Arch Chir 1987;371:207-16. [Crossref] [PubMed]
- Zhai C, Liu Y, Li W, et al. A comparison of short-term outcomes between Ivor-Lewis and McKeown minimally invasive esophagectomy. J Thorac Dis 2015;7:2352-8. [PubMed]
- Bizekis C, Kent MS, Luketich JD, et al. Initial experience with minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2006;82:402-6; discussion 406-7. [Crossref] [PubMed]
- Wormuth JK, Heitmiller RF. Esophageal conduit necrosis. Thorac Surg Clin 2006;16:11-22. [Crossref] [PubMed]
- Abiri A, Tao A, LaRocca M, et al. Visual-perceptual mismatch in robotic surgery. Surg Endosc 2017;31:3271-8. [Crossref] [PubMed]
- Sarkaria IS, Bains MS, Finley DJ, et al. Intraoperative near-infrared fluorescence imaging as an adjunct to robotic-assisted minimally invasive esophagectomy. Innovations (Phila) 2014;9:391-3. [Crossref] [PubMed]


