Pregnancy in adults with repaired/unrepaired atrial septal defect
Introduction
Atrial septal defect (ASD) is the most prevalent type of congenital heart disease and one of the most commonly recognized congenital cardiac anomalies that present in adulthood. It is characterized by a defect at the atrial level that allows pulmonary venous return to directly enter the right atrium. ASDs are classified into four types: ostium secundum (75% of cases), ostium primum (15–20% of cases), and sinus venosus (5–10% of cases) and, less commonly, an unroofed coronary sinus (1). The overall prevalence of ASD has been estimated to be 0.85 per 1,000 adults, which is likely an underestimate considering that some patients remain clinically asymptomatic, with a normal life expectancy (2). From a physiological perspective, the left-to-right shunt between systemic and pulmonary circulations leads to right ventricular (RV) volume overload with excessive pulmonary blood flow. Consequently, sequelae and complications include exercise intolerance, pulmonary vascular disease, RV dysfunction, paradoxical thromboemboli, and atrial arrhythmias (3-5).
Pregnancy in women with ASDs is generally well tolerated and considered to be at low risk for maternal and fetal morbidity and mortality (6,7). Nevertheless, hemodynamic and hemostatic changes throughout gestation may unmask previously unrecognized heart disease during pregnancy and increase the risk for maternal, fetal and obstetrical complications. This review focuses on issues related to pregnancy in women with repaired and unrepaired ASDs (Table 1).

Full table
Physiological changes during pregnancy
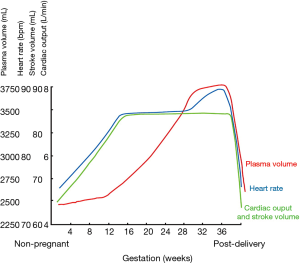
Several hemodynamic changes during a normal pregnancy contribute to the overall increase in cardiac workload (8-10). The two predominant changes consist of a reduction in vascular resistance and an increase in cardiac output. Hormonal alterations in early pregnancy lead to a reduction in both systemic and pulmonary vascular resistance, accompanied by a decrease in blood pressure by 5–10 mmHg in the first 2 trimesters. From the onset of pregnancy until the end of the second trimester, plasma volume increases progressively by up to 50% and is associated with a 20–30% rise in the red blood cell volume, resulting in relative anemia. Additionally, the increase in plasma volume initially contributes to a higher cardiac output, which peaks between 28 and 30 weeks of gestation. At this point, cardiac output may be 30–50% higher than the baseline pre-pregnancy value. The increase in cardiac output is driven by an initial increase in stroke volume and a heart rate that accelerates 10–20 bpm in the second trimester. Overall, oxygen consumption increases by 30% during a normal pregnancy.
During delivery, stroke volume increases by 300–500 mL with each contraction, which combined with pain-induced heart rate spikes, can result in an 80% increase in cardiac output when compared to pre-pregnancy values. Furthermore, blood loss differs according to mode of delivery and is less pronounced with vaginal births (~500 mL) compared to caesarean sections (~1,000 mL). Hemodynamic parameters gradually return to normal during a 4–6-week postpartum period (Figure 1) (11).
Normal pregnancy is also accompanied by changes to coagulation and fibrinolytic systems. These include increases in a number of clotting factors (e.g., VII, VIII, IX, X, XII and von Willebrand factor), fibrinogen and platelet activation, a reduction in protein S and inhibition of fibrinolysis. The latter is mediated by an increase in plasminogen activator inhibitor-1 and inhibitors of plasmin formation (e.g., α2 anti-plasmin and α2 macroglobulin). These changes underlie the hypercoagulable state associated with pregnancy, along with the increased risk for thromboemboli (12). Thromboembolic risk increases 5-fold in the third trimester, peaks in the early postpartum period, and remains elevated up to 6 weeks after delivery (13).
In patients with unrepaired ASDs, these pregnancy-induced physiological changes can aggravate RV volume overload, with the potential to trigger heart failure and contribute to atrial dilation and the genesis of atrial arrhythmias (i.e., atrial fibrillation and flutter). The reduction in systemic vascular resistance can favor transient pulmonary hypertension, with potential for shunt reversal, especially in the setting of pre-existing elevated pulmonary arterial pressures. This can result in a reduction in oxygenated blood in maternal and fetal circulations (14). Moreover, the hypercoagulable state combined with the potential for shunt reversal can increase the risk for paradoxical embolism.
Maternal outcomes in repaired and unrepaired ASD
Thromboembolic events
In addition to the risk of stroke related to arrhythmias (15) or incomplete prosthetic device endothelialization following percutaneous closure (16), patients with ASDs are likewise susceptible to paradoxical emboli. Paradoxical embolism is defined as the embolic migration of thrombus into the systemic circulation through a right-to-left shunt. The exact prevalence is unknown in the general ASD population. Paradoxical emboli have been shown to occur in patients with smaller defects and to be associated with deep venous thrombosis (5,17,18). In women without congenital heart disease, coagulation changes combined with venous stasis in the lower extremities contribute to the 1 to 2 per 1,000 risk of venous thromboembolism (13). These observations are consistent with the higher risk of paradoxical embolism observed during pregnancy in patients with unrepaired ASDs, reported to be between 2% and 5% (19,20). Although routine thromboprophylaxis is not recommended during pregnancy in patients with ASDs, clinical suspicion for deep venous thrombosis should incite prompt investigation considering the association with paradoxical emboli.
Arrhythmias
Atrial fibrillation and flutter are more prevalent in patients with unrepaired ASDs, or those with ASDs closed at an older age, when compared to the general population (15,20). It has been estimated that 10% of patients with unrepaired ASDs develop supraventricular arrhythmias, primarily atrial fibrillation, by 40 years of age (21,22). Adults with repaired ASDs remain at risk for atrial arrhythmias following surgical or percutaneous closure, with a higher risk in those with preceding arrhythmias (23-27). Overt symptomatic arrhythmias frequently occur during pregnancy in women with ASDs. Indeed, arrhythmias may be aggravated by the volume overload state associated with pregnancy, with its attendant electrophysiological and structural atrial remodelling effects.
In a retrospective study of 188 pregnant women with unrepaired (N=133) or repaired (N=55) ASDs, the most common cardiac complication was arrhythmia deemed to be of clinical significance, with an incidence of 4.3% (28). These included episodes of atrial fibrillation, sustained supraventricular tachycardia with syncope, and bouts of non-sustained ventricular tachycardia. No differences in arrhythmic complications were observed according to whether the ASD was repaired or unrepaired. However, in this particular study population, ASD repair was performed at a mean age of 26 years, with 14% and 18% of women with unrepaired and repaired ASDs, respectively, having had prior arrhythmias (28).
Evidence is scarce regarding the safety and efficacy of anti-arrhythmic and rate-slowing drugs during pregnancy. Challenges include achieving and maintaining stable drug levels in the context of hemodynamic, gastrointestinal, and metabolic changes. Two agents are considered relatively contraindicated, i.e., amiodarone and atenolol (29). Amiodarone has been associated with complications such as fetal hypothyroidism, goiter, premature birth, and a prolonged QT interval. Atenolol has been linked to fetal hypoglycemia, growth retardation, and bradycardia. Most other agents including digoxin, metoprolol, bisoprolol, propranolol, verapamil, diltiazem, adenosine, procainamide, quinidine, and flecainide have been insufficiently studied in humans but may be of benefit in selected patients despite potential risks and animal reproduction studies suggesting the possibility of adverse fetal effects. Animal studies have failed to demonstrate fetal risks associated with sotalol and lidocaine, although bradycardia can occur with either agent. In the event that arrhythmia is associated with hemodynamic instability, prompt electrical cardioversion should be considered (6,30). In women with ASDs and documented arrhythmias that are amenable to catheter ablation, procedures should ideally be performed prior to pregnancy. Under exceptional circumstances involving pregnant women with recurrent, refractory, and/or poorly tolerated supraventricular arrhythmias, catheter ablation with minimal or no fluoroscopy may be considered after carefully weighing potential risks and benefits (31).
Heart failure/pulmonary hypertension
Unless corrected in childhood, all forms of congenital heart disease in which a left-to-right shunt allows unrestricted volume and pressure overload to the pulmonary circulation can lead to pulmonary arterial hypertension. In comparison to patients with closed defects, those with unrepaired ASDs have a higher prevalence of increased pulmonary arterial pressures (35% vs. 13%), RV dysfunction (31% vs. 8%) and RV volume overload (18% vs. 1%) (32). These hemodynamic changes impair exercise capacity, even in patients considered asymptomatic, with exertional dyspnea noted in approximately 30% by the third decade of life (33-37). Pulmonary arterial hypertension is considered a late complication of an uncorrected ASD and uncommonly occurs before the age of 40. The prevalence has been estimated to be 10–35% in adults with secundum ASDs (38-40). However, the spectrum of pulmonary vascular disease is wide. Some adults with ASDs have only mild pulmonary vascular disease despite large shunts, whereas others develop severe irreversible pulmonary vascular disease, shunt reversal and chronic cyanosis (i.e., Eisenmenger syndrome) (4,41-44).
During pregnancy, the reduction in systemic vascular resistance associated with increased cardiac output can worsen right-to-left shunting and hypoxia. This may provoke pulmonary vasoconstriction and lead to refractory right heart failure. The impaired ability to adapt to vascular changes further contributes to poor maternal and fetal outcomes when pulmonary hypertension is present in women with congenital heart disease. In the late 1990s, studies reported maternal and fetal mortality rates up to 50% and 60%, respectively (45). More recent studies suggest that these rates are far lower (i.e., 5% and 2–13%, respectively), although maternal and fetal morbidity remain high (i.e., 30–50% and 20–40%, respectively) (46-48). Moreover, the incidence of premature delivery is also increased in this population. Thus, based on such reports, patients with ASD and pulmonary hypertension should be counselled against pregnancy (6).
Endocarditis
Infective endocarditis is a rare complication following ASD closure (49-51). It has been described up to 7 years after the procedure. Incomplete prosthetic device endothelialization has been proposed to explain late infections after percutaneous ASD closure. Currently, endocarditis prophylaxis is recommended for 6 months after patch or device closure if there is no evidence of a residual shunt (52,53). To our knowledge, only one case of endocarditis has specifically been described in a pregnant woman with an ASD, although the details surrounding the case are unclear, including site of infection, putative infectious agent, and associated abnormalities (19). Antibiotic prophylaxis is not recommended during delivery.
Obstetrical and fetal outcomes in repaired and unrepaired ASD
With the exception of patients with concomitant pulmonary hypertension, pregnancy is generally well tolerated in women with ASDs, with 85% completing their pregnancies. Nevertheless, small case control series suggest that women with unrepaired ASDs have a higher risk of neonatal events compared to those with repaired ASDs (28). Pregnancy with an unrepaired ASD appears to be associated with a higher risk of preeclampsia (7%; odds ratio 3.5), small for gestational age birth weight (21%; odds ratio 2.0) and fetal/perinatal mortality (2–3%; odds ratio 2.1) (19). Indeed, left-to-right shunts reduce the capacity to increase cardiac output and, consequently, placental perfusion. In contrast, neonatal outcomes in women with repaired ASDs are comparable to the general population. Limited evidence suggests that obstetrical complications and modes of delivery are similar in women with repaired and unrepaired ASDs (28).
ASD closure
Closure of an ASD is rarely required during pregnancy. Transcatheter closure of an ostium secundum ASD may be considered in pregnant women with cyanosis because of right-to-left shunting and poor fetal growth. However, this should not be performed in the context of an elevated pulmonary vascular resistance. Small case series have reported percutaneous ASD closure during pregnancy with good maternal and fetal outcomes (54-56). In these studies, indications for closure were heart failure with non-severe pulmonary hypertension, worsening functional class and recurrent stroke. Radiation exposure to the mother and fetus is a critical safety issue. To minimize risks to the developing fetus, when indicated, percutaneous ASD closure should be performed during the 2nd trimester under echocardiographic guidance with as little fluoroscopy exposure as possible (57). Transcatheter ASD closure under transesophageal echocardiographic guidance without fluoroscopy has been reported (58). Transthoracic or intracardiac echocardiographic guidance may be considered as alternative imaging modalities to avoid risks related to general anaesthesia. The rare case of surgical ASD closure during pregnancy has also been described. Cardiopulmonary bypass during pregnancy does not generally increase maternal mortality risk but is associated with an up to 10–15% fetal mortality risk. Indeed, continuous flow and hypothermia may lead to uterine contractions and a reduction in placental flow (59,60). Therefore, surgical ASD closure should be avoided during pregnancy and delayed until after delivery whenever possible.
Risk of congenital heart disease recurrence in the offspring
While most ASDs occur sporadically and are isolated, they may be associated with genetic mutations (TBX5, GATA4 and NKX2.5) (61,62). In the absence of a maternal genetic syndrome associated with the ASD, the incidence of congenital heart disease in the offspring is 2–4%, which is higher than the 0.6–0.8% baseline incidence in the general population (19).
Conclusions
Pregnancy in women with ASDs is generally well tolerated, with good maternal and fetal outcomes. Arrhythmias are the most common cardiac complication and occur in <5% of pregnant women. Nevertheless, an unrepaired ASD is associated with a higher risk of maternal and neonatal events. Although there is no definitive evidence demonstrating superiority, ASD closure may be considered prior to pregnancy. It is common in current practice to electively close asymptomatic but large and/or hemodynamically significant ASDs prior to childbearing years. Pregnancy remains contraindicated in patients with ASDs associated with severe pulmonary hypertension due to poor maternal and fetal outcomes. Cardiology follow up during pregnancy should be adapted to clinical symptoms.
Acknowledgements
Dr. P Khairy is supported by a research chair in electrophysiology and congenital heart disease.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 2008;118:e714-833. [Crossref] [PubMed]
- van der Bom T, Bouma BJ, Meijboom FJ, et al. The prevalence of adult congenital heart disease, results from a systematic review and evidence based calculation. Am Heart J 2012;164:568-75. [Crossref] [PubMed]
- Hannoush H, Tamim H, Younes H, et al. Patterns of congenital heart disease in unoperated adults: a 20-year experience in a developing country. Clin Cardiol 2004;27:236-40. [Crossref] [PubMed]
- Craig RJ, Selzer A. Natural history and prognosis of atrial septal defect. Circulation 1968;37:805-15. [Crossref] [PubMed]
- Rosas M, Attie F, Sandoval J, et al. Atrial septal defect in adults > or =40 years old: negative impact of low arterial oxygen saturation. Int J Cardiol 2004;93:145-55. [Crossref] [PubMed]
- Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011;32:3147-97. [Crossref] [PubMed]
- Canobbio MM, Warnes CA, Aboulhosn J, et al. Management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017;135:e50-e87. [Crossref] [PubMed]
- Pritchard JA. Changes in the blood volume during pregnancy and delivery. Anesthesiology 1965;26:393-9. [Crossref] [PubMed]
- Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin 2012;30:317-29. [Crossref] [PubMed]
- Robson SC, Hunter S, Boys RJ, et al. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 1989;256:H1060-5. [PubMed]
- Thorne SA. Pregnancy in heart disease. Heart 2004;90:450-6. [Crossref] [PubMed]
- Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol 2003;16:153-68. [Crossref] [PubMed]
- Toglia MR, Weg JG. Venous thromboembolism during pregnancy. N Engl J Med 1996;335:108-14. [Crossref] [PubMed]
- Fujitani S, Baldisseri MR. Hemodynamic assessment in a pregnant and peripartum patient. Crit Care Med 2005;33:S354-61. [Crossref] [PubMed]
- Karunanithi Z, Nyboe C, Hjortdal VE. Long-term risk of atrial fibrillation and stroke in patients with atrial septal defect diagnosed in childhood. Am J Cardiol 2017;119:461-5. [Crossref] [PubMed]
- Abaci A, Unlu S, Alsancak Y, et al. Short and long term complications of device closure of atrial septal defect and patent foramen ovale: meta-analysis of 28,142 patients from 203 studies. Catheter Cardiovasc Interv 2013;82:1123-38. [Crossref] [PubMed]
- Bannan A, Shen R, Silvestry FE, et al. Characteristics of adult patients with atrial septal defects presenting with paradoxical embolism. Catheter Cardiovasc Interv 2009;74:1066-9. [Crossref] [PubMed]
- Rigatelli G, Dell'avvocata F, Tarantini G, et al. Clinical, hemodynamic, and intracardiac echocardiographic characteristics of secundum atrial septal defects-related paradoxical embolism in adulthood. J Interv Cardiol 2014;27:542-7. [Crossref] [PubMed]
- Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol 2007;49:2303-11. [Crossref] [PubMed]
- Zuber M, Gautschi N, Oechslin E, et al. Outcome of pregnancy in women with congenital shunt lesions. Heart 1999;81:271-5. [Crossref] [PubMed]
- Vecht JA, Saso S, Rao C, et al. Atrial septal defect closure is associated with a reduced prevalence of atrial tachyarrhythmia in the short to medium term: a systematic review and meta-analysis. Heart 2010;96:1789-97. [Crossref] [PubMed]
- Giardini A, Donti A, Sciarra F, et al. Long-term incidence of atrial fibrillation and flutter after transcatheter atrial septal defect closure in adults. Int J Cardiol 2009;134:47-51. [Crossref] [PubMed]
- Gatzoulis MA, Freeman MA, Siu SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med 1999;340:839-46. [Crossref] [PubMed]
- Murphy JG, Gersh BJ, McGoon MD, et al. Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. N Engl J Med 1990;323:1645-50. [Crossref] [PubMed]
- Silversides CK, Haberer K, Siu SC, et al. Predictors of atrial arrhythmias after device closure of secundum type atrial septal defects in adults. Am J Cardiol 2008;101:683-7. [Crossref] [PubMed]
- Van De Bruaene A, Delcroix M, Pasquet A, et al. The importance of pulmonary artery pressures on late atrial arrhythmia in transcatheter and surgically closed ASD type secundum. Int J Cardiol 2011;152:192-5. [Crossref] [PubMed]
- Van De Bruaene A, Moons P, Belmans A, et al. Predictive model for late atrial arrhythmia after closure of an atrial septal defect. Int J Cardiol 2013;164:318-22. [Crossref] [PubMed]
- Yap SC, Drenthen W, Meijboom FJ, et al. Comparison of pregnancy outcomes in women with repaired versus unrepaired atrial septal defect. BJOG 2009;116:1593-601. [Crossref] [PubMed]
- Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol 2014;7:961-7. [Crossref] [PubMed]
- Ferrero S, Colombo BM, Ragni N. Maternal arrhythmias during pregnancy. Arch Gynecol Obstet 2004;269:244-53. [Crossref] [PubMed]
- Koźluk E, Piatkowska A, Kiliszek M, et al. Catheter ablation of cardiac arrhythmias in pregnancy without fluoroscopy: A case control retrospective study. Adv Clin Exp Med 2017;26:129-34. [Crossref] [PubMed]
- Engelfriet P, Meijboom F, Boersma E, et al. Repaired and open atrial septal defects type II in adulthood: an epidemiological study of a large European cohort. Int J Cardiol 2008;126:379-85. [Crossref] [PubMed]
- Campbell M. Natural history of atrial septal defect. Br Heart J 1970;32:820-6. [Crossref] [PubMed]
- Giardini A, Donti A, Specchia S, et al. Recovery kinetics of oxygen uptake is prolonged in adults with an atrial septal defect and improves after transcatheter closure. Am Heart J 2004;147:910-4. [Crossref] [PubMed]
- Giardini A, Donti A, Specchia S, et al. Long-term impact of transcatheter atrial septal defect closure in adults on cardiac function and exercise capacity. Int J Cardiol 2008;124:179-82. [Crossref] [PubMed]
- Brochu MC, Baril JF, Dore A, et al. Improvement in exercise capacity in asymptomatic and mildly symptomatic adults after atrial septal defect percutaneous closure. Circulation 2002;106:1821-6. [Crossref] [PubMed]
- Van De Bruaene A, Buys R, Vanhees L, et al. Cardiopulmonary exercise testing and SF-36 in patients with atrial septal defect type secundum. J Cardiopulm Rehabil Prev 2011;31:308-15. [Crossref] [PubMed]
- Gabriels C, De Meester P, Pasquet A, et al. A different view on predictors of pulmonary hypertension in secundum atrial septal defect. Int J Cardiol 2014;176:833-40. [Crossref] [PubMed]
- Vogel M, Berger F, Kramer A, et al. Incidence of secondary pulmonary hypertension in adults with atrial septal or sinus venosus defects. Heart 1999;82:30-3. [Crossref] [PubMed]
- Yong G, Khairy P, De Guise P, et al. Pulmonary arterial hypertension in patients with transcatheter closure of secundum atrial septal defects: a longitudinal study. Circ Cardiovasc Interv 2009;2:455-62. [Crossref] [PubMed]
- Sachweh JS, Daebritz SH, Hermanns B, et al. Hypertensive pulmonary vascular disease in adults with secundum or sinus venosus atrial septal defect. Ann Thorac Surg 2006;81:207-13. [Crossref] [PubMed]
- Mulder BJ. Changing demographics of pulmonary arterial hypertension in congenital heart disease. Eur Respir Rev 2010;19:308-13. [Crossref] [PubMed]
- Engelfriet PM, Duffels MG, Moller T, et al. Pulmonary arterial hypertension in adults born with a heart septal defect: the Euro Heart Survey on adult congenital heart disease. Heart 2007;93:682-7. [Crossref] [PubMed]
- Diller GP, Gatzoulis MA. Pulmonary vascular disease in adults with congenital heart disease. Circulation 2007;115:1039-50. [Crossref] [PubMed]
- Weiss BM, Zemp L, Seifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol 1998;31:1650-7. [Crossref] [PubMed]
- Ladouceur M, Benoit L, Radojevic J, et al. Pregnancy outcomes in patients with pulmonary arterial hypertension associated with congenital heart disease. Heart 2017;103:287-92. [Crossref] [PubMed]
- Curry RA, Fletcher C, Gelson E, et al. Pulmonary hypertension and pregnancy--a review of 12 pregnancies in nine women. BJOG 2012;119:752-61. [Crossref] [PubMed]
- Sliwa K, van Hagen IM, Budts W, et al. Pulmonary hypertension and pregnancy outcomes: data from the Registry Of Pregnancy and Cardiac Disease (ROPAC) of the European Society of Cardiology. Eur J Heart Fail 2016;18:1119-28. [Crossref] [PubMed]
- Jalal Z, Hascoet S, Baruteau AE, et al. Long-term complications after transcatheter atrial septal defect closure: a review of the medical literature. Can J Cardiol 2016;32:1315.e11-1315.e18. [Crossref] [PubMed]
- Vaideeswar P, Mishra P, Nimbalkar M. Infective endocarditis of the Dacron patch-a report of 13 cases at autopsy. Cardiovasc Pathol 2011;20:e169-75. [Crossref] [PubMed]
- Zahr F, Katz WE, Toyoda Y, et al. Late bacterial endocarditis of an amplatzer atrial septal defect occluder device. Am J Cardiol 2010;105:279-80. [Crossref] [PubMed]
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc 2008;139 Suppl:3S-24S. [Crossref] [PubMed]
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. [Crossref] [PubMed]
- Krishnamoorthy S, Butt M, Lip GY. Asymptomatic hypoxia in a young pregnant lady--unusual presentation of atrial septal defect. Int J Cardiol 2010;143:e34-6. [Crossref] [PubMed]
- Manivannan S, Dadlani G, Parsons M, et al. Surgical repair of atrial septal defect with severe pulmonary hypertension during pregnancy: a case report with literature review. Cardiol Young 2012;22:493-8. [Crossref] [PubMed]
- Soydemir DF, Johnston T, Clarke B. Percutaneous closure of an atrial septal defect during pregnancy using an Amplatzer occlusion device. J Obstet Gynaecol 2005;25:715-6. [Crossref] [PubMed]
- Orchard E, Dix S, Wilson N, et al. Reducing ionizing radiation doses during cardiac interventions in pregnant women. Obstet Med 2012;5:108-11. [Crossref] [PubMed]
- Yang Y, Zhang W, Wu Q, et al. Transcatheter closure of atrial septal defects without fluoroscopy: a well-established procedure for alternative use in children. EuroIntervention 2016;12:e652-7. [Crossref] [PubMed]
- Arnoni RT, Arnoni AS, Bonini RC, et al. Risk factors associated with cardiac surgery during pregnancy. Ann Thorac Surg 2003;76:1605-8. [Crossref] [PubMed]
- Strickland RA, Oliver WC Jr, Chantigian RC, et al. Anesthesia, cardiopulmonary bypass, and the pregnant patient. Mayo Clin Proc 1991;66:411-29. [Crossref] [PubMed]
- Basson CT, Huang T, Lin RC, et al. Different TBX5 interactions in heart and limb defined by Holt-Oram syndrome mutations. Proc Natl Acad Sci U S A 1999;96:2919-24. [Crossref] [PubMed]
- Schott JJ, Benson DW, Basson CT, et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science 1998;281:108-11. [Crossref] [PubMed]


