Risk factors and prognosis value of venous thromboembolism in patients with advanced non-small cell lung cancer: a case-control study
Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication in cancer patients. Patients with malignancy may face a 4–7-fold risk of VTE when compared to those without, especially in the first 3 months after diagnosis (1-3). Among solid cancers, lung cancer has been reported to have the highest incidence of VTE. Early analyses have estimated VTE incidence rates in lung cancer patients to be between 1.4% and 7.0% (4).
Several risk factors for cancer-related VTE have been identified, including cancer type, patient characteristics and anti-cancer treatment. A retrospective study reported that in non-small cell lung cancer (NSCLC), VTE risk was higher in adenocarcinomas than in squamous cell carcinomas [hazard ratio (HR), 1.9–3.3] (4). In addition, the patient’s age, chronic co-morbidities, advanced stage, high body mass index (BMI), and high performance status (PS) were also associated with high risks (5). Cancer treatment, such as surgery, chemotherapy, and radiotherapy may contribute to the development of VTE (6). It has been reported that cancer patients with a history of thrombotic events were associated with a worse prognosis and a decreased quality of life.
Previous studies have analyzed lung cancer patient populations of different races in all stages of lung cancer, including both SCLC and NSCLC. In this case-control study, we focused on a cohort of patients with NSCLC in advanced stages (stage III–IV) and assessed the factors including clinical pathology, cancer treatment and blood biomarkers just before VTE to test the hypothesis that these markers may correlate with VTE.
Methods
Ethics approval for this study was obtained from the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (No. 2014-354). This study was deemed as a quality improvement activity, conducting an appropriate audit of a clinical service. Accordingly, written consent was not required as the data were routinely available within current clinical practice and were analyzed using non-identifiable data.
Studied population
Between March 2012 and May 2015, we retrospectively identified 1,560 patients diagnosed with NSCLC in an advanced or metastatic stage (stage III or IV) at the First Affiliated Hospital of Zhejiang University. All patients granted consent to have their medical records reviewed. All diagnoses were confirmed by pathology. Patients were included if they had a histological diagnosis of primary lung cancer accompanied with symptomatic or asymptomatic VTE. Only patients diagnosed with VTE within 1 month before or after diagnosis of cancer were included. Patients with histories of malignant tumor, acute myocardial infarction and acute cerebral infarction were excluded. DVT events were confirmed by venous ultrasound image or a CT venous angiogram. The diagnosis of PE was confirmed by a CT pulmonary angiogram or a ventilation-perfusion scan. The 7th edition of the tumor node metastasis staging system was used to stage the NSCLC (7).
Each VTE patient was matched to three patients without VTE based on gender, age, pathology, clinical stage and the time of diagnosis. The matched patients had the same gender, less-than-5-year-age gap, the same pathology and clinical stage as well as with 2-month diagnosis time. Demographic information along with patient characteristics, state of disease and PS were recorded based on the electronic health records at time the diagnosis was confirmed. Other hematological biomarkers, treatment and metabolic were also recorded within 2 weeks of the diagnosis of VTE or when the control groups were selected. Overall survival (OS) was measured from the date of cancer diagnosis to death or the cutoff time of follow-up in January 2016.
Statistical analysis
Continuous variables were summarized as medians with standard deviation and compared with a t-test. For categorical variables, a Chi-squared test was used to compare the subgroups. Multivariate logical regression was used to identify factors associated with VTE in patients with lung cancer. P values <0.05 were considered statistically significant. Kaplan-Meier curves were generated for OS. All analyses were carried out using SPSS software, version 23.0 (IBM, USA).
Results
Characteristics of the studied population
Among the 1,560 patients diagnosed histologically with NSCLC at locally advanced or metastatic stages (stage III or IV), 32 (2.0%) were diagnosed with VTE. The median age at diagnosis was 60 years, and there were 25 (1.6%) incidences of VTE in the first 6 months. Approximately half of the VTE patients were diagnosed with DVT (43.8%), followed by PE (37.4%), and PE plus DVT (18.8%). By stage at diagnosis, 44% presented with stage IIIA or IIIB, and 56% with stage IV disease; 29 cases were treated with warfarin and low molecular heparin; the other three patients were not treated with anticoagulants because of their severe condition.
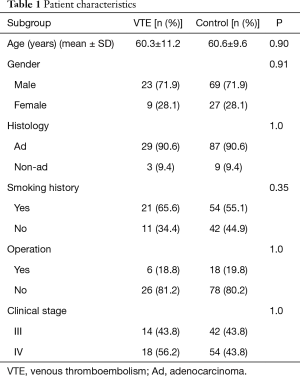
A total of 96 patients without VTE were selected from the NSCLC population as matching controls, including 69 males and 27 females, with an average age of 60.6±9.6 years. The baseline characteristics of patients with and without VTE are listed in Table 1. There were no significant differences in age, sex, gender, pathology and disease stage between the two categories.
Full table
Risk factors of VTE in lung cancer patient
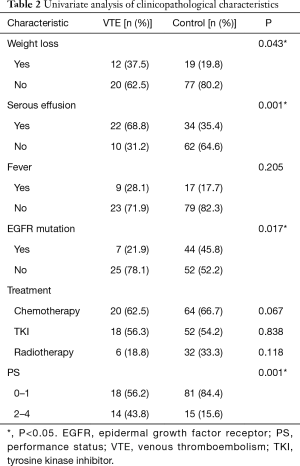
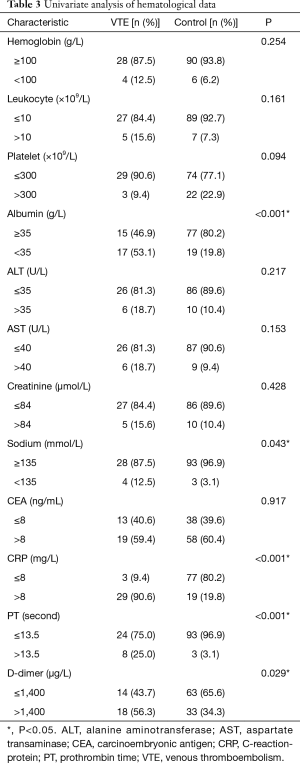
The clinicopathological characteristics were compared between the VTE and control groups (Table 2). Among the NSCLC patients, the factors associated with a significantly higher risk of VTE included weight loss, serous effusion, absence of the epidermal growth factor receptor (EGFR) mutation and poor PS. Furthermore, we have not found out the correlation of thrombosis risk with antitumor therapy when chemotherapy, EGFR-tyrosine kinase inhibitor (TKI) or radiotherapy were studied respectively. The comparative studies of hematological results between the VTE and control patients showed that the levels of albumin, sodium, C-reaction-protein (CRP), prothrombin time (PT) and D-dimer were associated with an increased risk of VTE (Table 3).
Full table
Full table
To screen for the activating gene mutation, all patients enrolled were analyzed for the presence of the EGFR mutation, 71 patients were analyzed for the anaplastic lymphoma kinase (ALK) mutation and 20 were analyzed for the ROS-1 mutation. Significant differences were found between the VTE and control groups for EGFR (7/32 vs. 44/96, P=0.017), but not for ALK (1/14 vs. 5/57, P=0.844) or ROS-1 (2/4 vs. 3/16, P=0.197).
Subsequently, we performed a multivariate logistic regression analysis that included weight loss, serous effusion, EGFR mutation, PS, leukocytes, platelet, albumin, serum sodium, CRP, PT, atrial fibrillation and D-dimer levels to identify the factors associated with VTE. This analysis revealed that weight loss (vs. no weight loss, OR =9.727, 95% CI: 1.370–69.070; P=0.023), poor PS (vs. normal PS, OR =5.790, 95% CI: 1.005–33.353; P=0.049), increased CRP (vs. normal CPR, OR =38.551, 95% CI: 5.511–269.679; P<0.001) and long PT (vs. normal PT, OR =43.958, 95% CI: 2.400–805.025; P=0.011) were significantly associated with a high risk of VTE.
Impact of VTE on survival
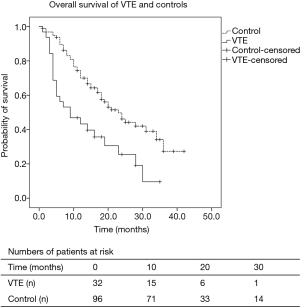
Figure 1 shows the Kaplan-Meier survival curves of patients in the two groups. It shows that VTE patients were associated with worse OS than patients without VTE (Figure 1), with a median OS of 14.2 months (95% CI: 10.0–18.3) vs. 24.4 months [95% CI: 21.3–27.6); P<0.001] in the locally advanced or metastatic stage. It also shows the number of patients at risk at different time points.
Discussion
In this study, we aimed at detecting the incidence, risk factors and prognosis of VTE in the patients with advanced or metastatic stage with NSCLC. Of the 32 VTE patients, 78% of the thrombosis occurred within half a year after the diagnosis of lung cancer, which is consistent with previous research (8). In the patients with stage III–IV, the prevalence rates of DVT, PE, and PE plus DVT were 43.8%, 37.4% and 18.8%, respectively. Investigation in a recent study reported that the incidence of VTE among NSCLC patients was 3.0–13.8% (9-11). The reason that the results in our study were lower than previous reports may be accounted for by the Chinese patients studied, as the Asian ethnicity is found to be associated with a 60% lower risk of VTE in NSCLC (12).
The incidence of VTE in cancer patients varies according to the histological type of cancer and its stage. It was demonstrated that adenocarcinoma histology was an independent risk factor for increased risk of VTE in metastatic NSCLC (6). In a previous study of 91,933 patients, the 2-year cumulated incidence rates of VTE in adenocarcinomas, squamous cell carcinomas and large cell carcinomas were 5%, 2.6% and 3.2%, respectively (4). In addition, advanced and metastatic cancer was shown to be associated with an increased risk of VTE when compared with localized tumors (13). In the current study, the pathology type and stage of tumor were used as balance factors between the VTE and control groups.
In our series, we identified that patients with weight loss, serous effusion, poor PS, as well as hypoalbuminemia and hyponatremia may show increased risks of VTE. This finding is in agreement with previously published reports (14). These risk factors may have some connections. For example, weight loss, hypoalbuminemia and hyponatremia are symptoms of body consumption, which are associated with poor PS. We found that anti-tumor therapy did not increase the risk of thrombosis, which is inconsistent with earlier studies (15,16). It maybe because we have not explored the specific treatment process, such as chemotherapy drugs, treatment course, the number of radiotherapy and TKI dose of drugs.
A high level of CRP is also considered an additional risk factor of VTE in NSCLC patients. In recent research, leukocytosis was significantly associated with an increased risk of developing DVT in lung cancer patients (10). CRP, along with leukocytosis, was strongly associated with inflammation, which resulted in an increase of thrombosis. Possibly due to the systemic therapy or stage, there was no significant difference of leukocytes between the two groups.
We observed that long PT and high levels of D-dimer were associated with an increased risk of VTE, while the platelet count showed little effect. Several studies identified D-dimer reflects the activation of the blood coagulation system, and high D-dimer levels were also associated with a poor response to the anticancer treatment (17). Studies have shown that malignant cells aberrantly express tissue factor and may be involved in the induction of cancer-associated thrombosis (13,18).
In this study, an elevated level of carcinoembryonic antigen (CEA) was not related to VTE in lung cancer patients. A previous meta-analysis indicated that the serum CEA level carried prognostic and predictive information for death risk in NSCLC patients, independent of treatment (19). The patients we included were at an advanced or metastatic stage, and most of them had high levels of CEA.
The presence of activating gene mutations in EGFR, ALK and ROS-1 were analyzed. EGFR mutation was associated with VTE development, but ALK re-arrangement and ROS-1 mutations were not associated with VTE development. The mechanism is not clear. Preclinical data have demonstrated that EGFR inhibition reduces the expression of tissue factor, which regulates the tumor pro-coagulant activity (20). Therefore, the patients who have an EGFR mutation treated with TKI may show a reduced incidence of VTE. According to the study conducted by Lee (6), the treatment of EGFR TKI was associated with a 60% increased risk of VTE. However, in our study, three patients with rare gene mutations (one ALK positive and two ROS-1 positive) and VTE complications have reached remission after being treated with a TKI (Crizotinib). Additionally, the previous study suggested that K-RAS was a driver of thromboembolic risk and was involved in the pathophysiology of cancer-associated thrombosis (21), but only a small portion of the patients took the K-RAS test in our study.
Our study demonstrated that cancer patients with VTE complications have a reduced survival rate compared with those without VTE complications. It is difficult to infer causality for this finding. It could be explained by the increased deaths attributable to VTE or to the disease severity. The Cox regression identified that weight loss, poor PS and an elevated level of D-dimer indicated a poor prognosis for NSCLC.
This analysis has several limitations. First, the unrecognized bias from the retrospective nature of the study and the single center of the study limits the interpretation of the results. The follow-up period used may be too short to demonstrate a realistic outcome. In addition, approximately 5% of the patients were lost during various stages of this study, which could underestimate the real survival time. Finally, the prevalence of DVT was mostly checked by venous ultrasound, which is insensitive to detecting distal DVT.
Conclusions
Our study used case-control to balance the gender, age, pathology, clinical stage and the time of diagnosis in the VTE and control groups. We discussed the risk factors of VTE and the prognosis of lung cancer patients combined with VTE. Furthermore, the effects of gene mutation, TKI therapy and anticoagulant treatment in VTE patients remain unknown. Further prospective observational research is needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (No. 2014-354).
References
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003;107:I17-21. [Crossref] [PubMed]
- Khorana AA, Francis CW, Culakova E, et al. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 2005;104:2822-9. [Crossref] [PubMed]
- Kyriazi V, Theodoulou E. Assessing the risk and prognosis of thrombotic complications in cancer patients. Arch Pathol Lab Med 2013;137:1286-95. [Crossref] [PubMed]
- Chew HK, Davies AM, Wun T, et al. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost 2008;6:601-8. [Crossref] [PubMed]
- Agnelli G, Verso M, Mandalà M, et al. A prospective study on survival in cancer patients with and without venous thromboembolism. Intern Emerg Med 2014;9:559-67. [PubMed]
- Lee YG, Kim I, Lee E, et al. Risk factors and prognostic impact of venous thromboembolism in Asian patients with non-small cell lung cancer. Thromb Haemost 2014;111:1112-20. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Li R, Hermann G, Baldini E, et al. Advanced nodal stage predicts venous thromboembolism in patients with locally advanced non-small cell lung cancer. Lung Cancer 2016;96:41-7. [Crossref] [PubMed]
- Kourelis TV, Wysokinska EM, Wang Y, et al. Early venous thromboembolic events are associated with worse prognosis in patients with lung cancer. Lung Cancer 2014;86:358-62. [Crossref] [PubMed]
- Zhang Y, Yang Y, Chen W, et al. Prevalence and associations of VTE in patients with newly diagnosed lung cancer. Chest 2014;146:650-8. [Crossref] [PubMed]
- Vitale C, D'Amato M, Calabrò P, et al. Venous thromboembolism and lung cancer: a review. Multidiscip Respir Med 2015;10:28. [Crossref] [PubMed]
- Ay C, Ünal UK. Epidemiology and risk factors for venous thromboembolism in lung cancer. Curr Opin Oncol 2016;28:145-9. [Crossref] [PubMed]
- Falanga A. The incidence and risk of venous thromboembolism associated with cancer and nonsurgical cancer treatment. Cancer Invest 2009;27:105-15. [Crossref] [PubMed]
- Wang Z, Yan HH, Yang JJ, et al. Venous thromboembolism risk factors in Chinese non-small cell lung cancer patients. Support Care Cancer 2015;23:635-41. [Crossref] [PubMed]
- Mellema WW, van der Hoek D, Postmus PE, et al. Retrospective evaluation of thromboembolic events in patients with non-small cell lung cancer treated with platinum-based chemotherapy. Lung Cancer 2014;86:73-7. [Crossref] [PubMed]
- Walker AJ, Baldwin DR, Card TR, et al. Risk of venous thromboembolism in people with lung cancer: a cohort study using linked UK healthcare data. Br J Cancer 2017;116:e1. [Crossref] [PubMed]
- Ay C, Vormittag R, Dunkler D, et al. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol 2009;27:4124-9. [Crossref] [PubMed]
- Davila M, Amirkhosravi A, Coll E, et al. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost 2008;6:1517-24. [Crossref] [PubMed]
- Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012;76:138-43. [Crossref] [PubMed]
- Yu JL, May L, Lhotak V, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood 2005;105:1734-41. [Crossref] [PubMed]
- Corrales-Rodriguez L, Soulières D, Weng X, et al. Mutations in NSCLC and their link with lung cancer-associated thrombosis: a case-control study. Thromb Res 2014;133:48-51. [Crossref] [PubMed]