Why anti-PD1/PDL1 therapy is so effective? Another piece in the puzzle
Immune checkpoint inhibitors anti-programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) are currently changing the approach of treatment of non-small cell lung cancer patients (NSCLCs). During the last 2 years, the anti-PD-1 inhibitors, nivolumab (OPDIVO, Bristol-Myers Squibb) and pembrolizumab (KEYTRUDA, Merck Sharp and Dohme Corporation) and the anti-PD-L1 inhibitor atezolizumab (TECENTRIQ, Genentech Oncology) have been approved by the U.S. Food and Drug Administration (FDA) in the treatment of patients with advanced NSCLC with progression on or after first-line therapy. Indeed, the European Medicines Agency (EMA) has endorsed Nivolumab and Pembrolizumab for the same indication. In recent times, pembrolizumab has also been recommended by both the U.S. and European agencies for the first-line therapy of NSCLCs with advanced disease. Furthermore, two other drugs as durvalumab (MEDI4736, AstraZeneca) and avelumab (MSB0010718C, Merck KGaA & Pfizer) are being examined for the treatment of NSCLC patients (1).
These great achievements in lung cancer therapy follow years of active research that revealed the intimate interplay between tumor cells and the immune system.
As well established, tumor cells are able to avoid control and destruction by the immune system using a range of complex and often overlapping mechanisms that lead to disruption of key components involved in the effective antitumor response.
The adaptability and the specific structure of the immune system allows the discrimination between self from non-self and lets to assault foreign pathogens and infected self-tissues. Non-specific first line barrier is the role of the innate system. It contains a large amount of components, including antigen-presenting cells (APCs), dendritic cells, mastocytes, histiocytes, and macrophages. On the contrary, the adaptive immune system induces the production of helper CD4+ T-cells, cytotoxic CD8+ T-cells and antibody-releasing plasma cells.
Phagocytosis by the mononuclear phagocyte system (MPS) is one of the main processes involved in the innate immunity. The two major cell types of the MPS are monocytes, which differentiate to macrophages when exiting circulation to enter tissues and macrophages, present in all tissue. Macrophages have a uniquely efficient capacity to phagocytose multiple targets, including some types of diseased cells among healthy cells. However, macrophages fail to perceive and attack tumors despite their foreign genomes (2). There are two principal phenotypes of macrophages that are two different phases of polarized macrophages inducted by cytokine panels of T helper cells: M1 and M2. The M1 phenotype is activated by Thelper1 cells when microbial agents such as LPS (lipopolysaccharide) and cytokines [interferon gamma (IFN-γ), TNF-α, IL-12, etc.] are released. M1 macrophages have the ability to eradicate tumor cells and are strong protectors against microbes.
On the contrary, M2 macrophages are a ‘differently triggered’ categories of macrophages made active by triggered Th2 cell–derived IL4. This system induces the delivery of an alternative panel of chemokines and cytokines that is opposite to the sheet of classically activated M1 macrophages.
The so-called M2 macrophages have also been reported as inflammation inhibitors and have been associated with tumor progression. The M2 macrophages lower the expression of iNOS, release anti-inflammatory cytokines such as IL-10 and decrease T cell proliferation, reducing antigen presentation. Macrophages within the tumour microenvironment (TME) have been called tumour associated macrophages (TAMs) and they are able to produce cytokines, inflammatory mediators, chemokines, several growth factors, and other molecules. Indeed, TAM is a misnomer if considered as giant cell that devours, since these cells seem to have lost most or all of their ability to phagocytose. They play dual roles and seem to be two-faced: in the early stage of tumors, they have anti-tumor features adopting the M1 phenotype. However, with late stage tumor, TAMs change into tumor-promoting M2-like phenotype. During tumor progression, M2 macrophages favor invasion of neoplastic cells by producing a large quantity of cytokines, growth factors and ECM-remodeling molecules and control neoplastic cells expanding, moving and angiogenesis.
In TME, the adaptive immune system plays a pivotal role in the fight of malignant cells. The adaptive immune system has the unique ability to develop highly specific responses through highly specific antigen receptors on B-cells (B-cell receptor) and T-cells (T-cell receptor). As an antigen binds the B- or T-cell receptor, the development of a strong antigen-specific immune response occurs together with the growth of long-lived memory cells. In the immune response against tumor cells, APCs introduce tumor antigens in the context of major histocompatibility complex class I and class II molecules and CD8+ and CD4+ T-cells recognize tumor antigens through a specific T cell receptor.
In lymphoid tissue, after the first APC-induced activation, CD8+ T-cells act in cell-mediated cytotoxicity and have the capability to destroy cells with modified self-antigens and cells that are identified as non self. CD8+ T-cells are important players in the immune response against tumor cells. On the other hand, CD4+ T-cells provide help for CD8+T cells and differentiate into various types of helper CD4+ T-cells through the release of specific cytokines. Co-stimulatory pathways such as CD28-B7-1 and CD28-B7-2 activate CD4+ and CD8+ T cell, inducing cytokines secretion, proliferation and the acquisition of cytolytic properties and ability to migrate to sites of TME.
Another key point in the fight of the immune system against tumor cells are the so-called immune checkpoints, specific receptors that either turn the signal off or on (inhibitory molecules or co-stimulatory molecules, respectively). Various type of cancers preserve themselves from the attack of the immune system by blocking the T cell signal. In particular, in TME, CD4+ T-cells acquire the differentiation markers of regulatory T-cells (Tregs), becoming crucial negative regulators of the immune system capable to block the host’s antitumor immune response. The immune response can also be blocked by cytotoxic T lymphocyte associated antigen 4 (CTLA4) that controls the CD4+ T cell function in lymphoid tissue. In TME, as activated T cells start to exhibit the coinhibitory PD1 receptor, CD4+ T helper and CD8+ T cytotoxic release IFN-γ which induces the macrophages tumor killing activity and the expression of PDL1 by tumor cells and macrophages (Figure 1). PD-1 also binds PD-L2 (B7-DC) which is present specifically on dendritic cells and macrophages. These particular pattern of expression, suggest that PD-L2 can be active in lymphoid organs whilst PD-L1 promotes self-tolerance in peripheral tissue. However, the role of PD-L2 in immunomodulation is not clearly established yet.
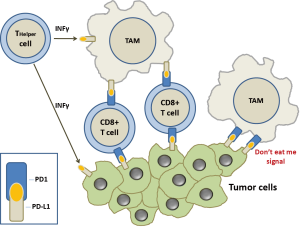
In TME, when PDL1+ cells meet tumor-specific PD1+/CD8+ T cells, these latter are functionally deactivated. Furthermore, CTLA4 expression by Treg cells inhibits the release of cytokines by CD8+ T cells and tumor cells killing.
Current cancer immunotherapies are based on overpassing this inhibition, either by local handling of immunoregulatory molecules in the TME, including immune checkpoints and by general activation of the immune system. The first molecule studied, ipilimumab, is a targeted drug against an immune checkpoint. It is a monoclonal antibody (MoAbs) that inhibits the binding of CTLA-4 receptor expressed on T cells with its ligands, B7-1 and B7-2, located on the surface of cells that present antigens but not on the surface of malignant cells. Subsequent studies have turned the attention to the PD-1:PD-L1/PD-L2 immunologic axis.
A better acknowledgment of cancer immunology has brought forth the development of several MoAbs which are able to reverse the immune response through the blockade of the PD-1/PD-L1 axis. Two different classes of MoAbs exist: the anti-PD-L1 and anti-PD1 MoAbs. Atezolizumab, Durvalumab, and Avelumab are anti PDL1 molecules, IgG1 isotypes with genetically modified Fc fragments, which block the PD-L1 and prevent its interaction with PD-1 receptor. On the other hand, the anti-PD-1 MoAbs, Nivolumab and Pembrolizumab are fully human and humanized respectively, IgG4 MoAbs. They block the binding between PD-1 receptor and its natural ligands, PD-L1 and PD-L2 (1). All these MoAbs have shown a very promising activity in clinical studies, achieving an overall response rates (ORR) of about 20% in unselected and in heavily pre-treated NSCLC patients (1).
The majority of the clinical results happen early, around 50% in the first eight weeks of treatment, and may be prolonged in time. Durable objective (partial or complete) responses following anti-PD1 therapy have been reached also in patients with advanced melanoma (31–44% of patients) and renal cell carcinoma (22–25%) with extended overall survival compared with conventional therapies.
Why anti-PD1/PDL1 therapy is so effective? The answer to this question may partly reside in the multifaceted role that TAMs exert in tumorigenic processes.
During the past decades, TAMs have been found ineffective in anticancer therapy, but recent data have perhaps changed this common view. The noting of TAMs in tumors has been observed from two centuries ago. Up to date, nevertheless, their anticancer role was usually overlooked. Although TMAs are the most effective cells involved in antitumor activity, it is clear that a large amount of solid cancers are wildly crowded with TAMs and that these cells are linked to worse clinical response. In fact, the high concentration of TAMs in a tumor seems to be correlated with poor prognosis, late tumor stage and high metastatic rate. A number of clinical trials have demonstrated a relationship between large quantity of TAMs and reduced prognosis because TMAs activity may also influence tumor development, progression and metastasis process (3).
In a previous study, Weissman, Gordon, and colleagues proved that the signal regulatory protein alpha (SIRPα)—a ‘don’t eat me’ receptor—is expressed on macrophages and binds CD47 “marker of self”, present on the surface of some cancer cells. The self-signaling is a powerful brake that overrides the phagocytosis process. Indeed, interaction arises between the SIRPα membrane receptor present on the macrophages surface as “don’t eat me marker” and CD47 membrane protein, defined as ubiquitous “marker of self” and present on the surface of candidate target cell (or particle).
CD47, broadly inhibits phagocytosis and is abundantly expressed on all healthy cells. That signaling ultimately turns off cytoskeletal myosin-II, which otherwise makes the very active process of engulfing a foreign cell or particle efficient. Myosin-II has a vital role in multiple, cytoskeletal-intensive activities of macrophages and phagocytosis is also favored by the stiffness of a cell or particle, and myosin-II has again been shown to be key.
So, inhibiting this signaling at various upstream or downstream points in the CD47-SIRPα pathway can likewise make engulfment of “self” cells more efficient. In fact, recently, it has been explained that macrophage phagocytic activity is made up of blocking the interaction between CD47 and SIRPα and this healing strategy is at present target of several scientific trials in cancer therapy (4).
Another important piece has recently been inserted in this complex puzzle. Gordon et al. have shown that both mouse and human TAMs expressed PD-1 and an M2-like surface profile (5). These Authors proved that PDL1 binding to macrophage PD1 acts as a “don’t eat me” signal and the expression of PD-1 on M2 macrophages is linked with decreased phagocytic activity (Figure 1). Indeed, it has been shown that the expression of PD-1 on TAMs is enhanced with late stage of disease in primary human cancers and along the time in mouse cancer models. Moreover, TAM PD-1 expression was associated with reduced phagocytic capability and blocking PD1/PDL1 axis seemed to be related in vitro to prolonged survival of mouse cancer cells and in vivo to increased TMA phagocytosis and reduced tumor growth in a macrophage-dependent way. PD-1 expression hinders different immune cell types in the TME, including dendritic cells, natural killer cells, B cells and T cells. Gordon et al. (5) expanded this concept including macrophages and proposing that PD-1 expression is a general system for blocking immunity along the adaptive and innate immune system. This conclusion has important therapeutic implications, in that with one stone (an anti-PD1 or PD-L1 inhibitor) two birds can be killed (innate and adaptive immune processes). This could further explain why immune checkpoint inhibitors against PD1 or PD-L1 are so effective in cancer patients, even if affected by very aggressive forms. Furthermore, bindings of SIRPα and PD-1 to CD47 and PD-L1 respectively, independently affect phagocytosis by TAMS. It has been shown that the block of both CD47 and PD-L1 may restore the phagocytic activity against tumor cells in vitro and in vivo. Therefore, it will become possible to develop TAMS-targeted therapies which could synergize with check point inhibitors.
One of the crucial points in the management of patients to be treated with immune checkpoint inhibitors is the accurate selection of tumors who will respond to these innovative treatments. Immunohistochemical (IHC) detection of PD-L1 is at the moment the most validated biomarker and it is currently used in clinical practice for the selection of NSCLC patients to be treated with pembrolizumab. The new findings reported by Gordon et al. (5) may also impact on the diagnostic process. Indeed, while patients affected by cancer are routinely treated with anti-PD-1/antiPD-L1 drugs, the effects of PD-1 blockage on TMAs in human malignancies cannot be underestimated. We suggest that the IHC assessment of PD1 in macrophages might help in the selection of patients for anti PD1/PD-L1 immunotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Marchetti A, Barberis M, Franco R, et al. Multicenter Comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) Assays to Test PD-L1 Expression for NSCLC Patients to Be Treated with Immune Checkpoint Inhibitors. J Thorac Oncol 2017;12:1654-63. [Crossref] [PubMed]
- Zheng X, Turkowski K, Mora J, et al. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget 2017;8:48436-52. [PubMed]
- Alvey C, Discher DE. Engineering macrophages to eat cancer: from "marker of self" CD47 and phagocytosis to differentiation. J Leukoc Biol 2017;102:31-40. [Crossref] [PubMed]
- Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012;109:6662-7. [Crossref] [PubMed]
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017;545:495-9. [Crossref] [PubMed]


