Intermittent chest tube clamping may shorten chest tube drainage and postoperative hospital stay after lung cancer surgery: a propensity score matching analysis
Introduction
Surgical resection is an effective treatment for early stage lung cancer. Chest tube insertion is required after this surgery. Shortening the duration of postoperative chest tube drainage in lung cancer patients may accelerate recovery, shorten hospital stay, and thereby decrease the economic burden on the healthcare system (1,2). Different chest tube removal protocols are applied in different medical centers, such as electronic chest drainage system (3), external suction (4), and rigorous protocol (5). Thus far, however, no standard protocol of chest tube management has been developed (6-9).
Chest tube clamping is performed before chest tube removal in patients with pneumothorax (10,11). In our practice, we have found that the amount of pleural effusion fluid drained decreases after chest tube clamping. We have therefore modified our chest tube management protocol from traditional gravity drainage to a protocol combining intermittent chest tube clamping with gravity drainage since January 2014. We designed this retrospective study with propensity score matching analysis to determine whether intermittent chest tube clamping reduces the duration of chest tube drainage and postoperative hospital stay after lung cancer surgery.
Methods
Patients
We retrospectively reviewed the medical data of patients with resectable lung cancer who underwent lobectomy and systematic mediastinal lymph node dissection in the Department of Thoracic Surgery II, Peking University Cancer Hospital between July 2012 and June 2016. Patients with the following conditions were excluded from this analysis because each of these conditions can dramatically affect the duration of chest tube drainage: bronchoplasty and/or pulmonary arterioplasty, prolonged air leakage, reoperation due to chylothorax, atelectasis, liver cirrhosis, renal insufficiency, wound infection, costectomy, or severe subcutaneous emphysema.
All patients underwent routine preoperative staging including computed tomography of the chest, brain magnetic resonance imaging, abdominal ultrasonography, and bone scintigraphy or positron-emission tomography/computed tomography. Fibrobronchoscopic biopsy was performed in patients with centrally located tumors. Pulmonary function testing and cardiac evaluation were also performed as part of the preoperative assessment.
The Institutional Review Board of Beijing Cancer Hospital approved the study (ID: 2017KT27), and patient consent was waived.
Chest tube management protocols
All patients underwent lateral thoracotomy or video-assisted thoracoscopic surgery (VATS) and were operated on by the same thoracic surgical team. No pleural tents or buttressed staple lines were used in this series. At the end of operation, the lung parenchyma was submerged in sterile saline to test for air leakage, and a single 24-Fr chest tube was placed in each patient.
The chest tube management protocol was modified in January 2014. Before that time, patients (control group) were managed with gravity drainage (water seal only, without suction). Since January 2014, patients (clamping group) were managed with gravity drainage during the first 12–24 h (depending on the time of surgery completion) after surgery. Once a radiograph confirmed re-expansion of the lung on the morning of the first postoperative day and no air leak was detected, the chest tube would be clamped, and the nurses would check the patient every 6 h. If the patient had no problems with compliance, the clamp was removed for half an hour in the morning to record the drainage volume every 24 h.
If patients developed intolerable abnormal symptoms, such as dyspnea, pneumothorax, and severe subcutaneous emphysema after chest tube clamping, the clamp would be removed for 30 min and reapplied after the symptoms had resolved. Such patients were placed under more rigorous surveillance after re-clamping, which required the medical staff to check on the patients every 2–4 h in order to promptly detect abnormal symptoms. This protocol was continued until another radiograph excluded the presence of pneumothorax. The chest tube clamping protocol in the clamping group has been illustrated in Figure 1.
The daily output of pleural fluid was recorded. The criteria for chest tube removal were as follows: (I) drainage volume <200 mL in 24 h; (II) absence of air leakage and intrathoracic hemorrhage, and (III) absence of signs of purulent pleural effusion and atelectasis.
Statistical analysis
All clinical data were recorded. The pleural drainage volume was recorded on each day after the surgery until chest tube removal. Multivariate logistic regression analyses were performed for binary outcomes in order to identify factors significantly associated with duration of chest tube drainage. Continuous variables were expressed as mean ± standard deviation (or median and interquartile ranges for non-normally distributed data). Categorical variables were expressed as frequencies and percentages. The Student t-test was used to analyze normally distributed data after confirming homogeneity by the Levene test. The Mann-Whitney U-test was used to analyze non-normally distributed data. The Pearson chi-square (χ2) test or the Fisher exact test was used to compare proportions, as required. The P values for differences were calculated with a significance level of P<0.05. Propensity scores included the following variables: sex, operation side, VATS, and chylothorax. These variables were tested as factors that potentially determine the duration of chest tube drainage through multivariate logistic regression analysis, representing the probability of being assigned to either the control group or the clamping group. We matched propensity scores 1:1 using nearest neighbor methods, no replacement, and 0.2 caliper width. SPSS software (version 20.0; SPSS, Chicago, IL, USA) was used for all analyses.
Results
Demographic and perioperative data
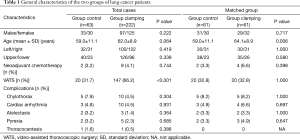
Between July 2012 and June 2016, a total of 327 lung cancer patients underwent lobectomy and systematic mediastinal lymph node dissection in our hospital. Of these, 42 patients were excluded from the analysis owing to the following reasons: bronchoplasty and/or pulmonary arterioplasty (n=27), prolonged air leakage (n=8), reoperation due to chylothorax (n=2), atelectasis (n=1), liver cirrhosis (n=1), renal insufficiency (n=1), wound infection (n=1), costectomy (n=1), and severe subcutaneous emphysema (n=1, combined with prolonged air leakage). Thus, finally, 285 consecutive patients with operable lung cancer treated using lobectomy and systematic mediastinal lymphadenectomy were retrospectively analyzed. There were 130 male patients and 155 female patients. The mean age of the patients was 61.3±9.6 years. The overall duration of chest tube drainage was 3.9±1.4 days, and the overall postoperative stay was 5.6±1.6 days. Before January 2014, 63 patients (control group) were managed with gravity drainage (water seal only, without suction), and after January 2014, 222 patients (clamping group) were managed with the intermittent clamping protocol.
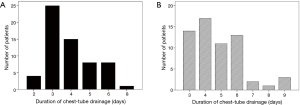
The rate of thoracocentesis after chest tube removal was not higher in the clamping group than in the control group (0.5% vs. 1.6%, P=0.386). The rates of pyrexia were also comparable in the two groups (2.3% vs. 3.2%, P=0.685). After propensity score matching, 61 matched patients remained in each group. The characteristics of both groups, except age, were compared again after propensity score matching (Table 1). The clamping group had a shorter duration of chest tube drainage (3.9±1.3 vs. 4.8±1.6 days, P=0.001) and shorter postoperative stay (5.7±1.8 vs. 6.4±1.8 days, P=0.025) than the control group. The duration of chest tube drainage was ≤4 days in 72.1% (44/61) of patients in the clamping group and 50.8% (31/61) of patients in the control group (Figure 2).
Full table
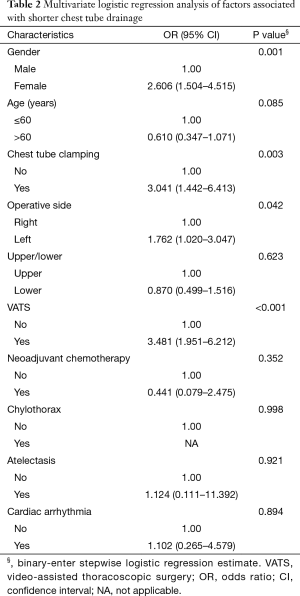
Multivariate logistic regression analysis of factors associated with duration of chest tube drainage
All variables, including age, sex, chest tube clamping, operation side, upper/lower lobectomy, VATS, neoadjuvant chemotherapy, chylothorax, atelectasis, and cardiac arrhythmia, were examined in multivariate logistic regression analysis models in the entire cohort. The study group was stratified into two subgroups according to the duration of chest tube drainage (<4 or ≥4 days). Our analysis revealed that female sex [95% confidence interval (CI): 1.504–4.515, P=0.001], chest tube clamping (95% CI: 1.442–6.413, P=0.003), left lobectomy (95% CI: 1.020–3.047, P=0.042), and VATS (95% CI: 1.951–6.212, P<0.001) were associated with a significantly shorter duration of chest tube drainage (Table 2).
Full table
Discussion
In pulmonary resection, the aims of chest tube insertion are to evacuate air and fluid from the pleural space, ensure complete pulmonary re-expansion, and restore respiratory mechanics (12). The criteria for chest tube removal include complete expansion of the remaining lung tissue, and cessation of air leakage and high-volume fluid drainage. Since chest tubes can exacerbate postoperative pain, cause ineffective ventilation, decrease sputum evacuation, and lead to atelectasis, thoracic surgeons aim to reduce the duration of chest tube drainage. In one study that examined the correlation between duration of chest tube drainage and pulmonary function after wedge resection, chest tube removal after 90 min postoperatively was found to be beneficial in terms of earlier respiratory rehabilitation, shorter hospital stays, and less analgesic use (5). However, it seems dangerous to remove a chest tube within a couple of hours after lobectomy, which is frequently performed in lung cancer patients. Thus, we had been working on finding a more reasonable method to reduce the duration of chest tube drainage in lung cancer patients, when we noticed the effect of tube clamping. To date, no study has investigated chest tube clamping for earlier chest tube removal after lobectomy for lung cancer.
The present study demonstrated that chest tube clamping decreased the duration of chest tube drainage. This finding may be explained by the revised Starling law (13). Pleural fluid is drained by an absorptive pressure gradient through the visceral pleura, by cellular mechanisms (14), and by lymphatic drainage, as the lymphatics open directly on the parietal pleura (lymphatic stomata) (15,16). According to the Starling equation, absorption and lymphatic drainage increase with increase in interstitial hydrostatic pressure, which occurs when the chest tube is clamped. This mechanism may explain how clamping facilitates chest tube removal.
Since thoracic drainage is intended to maintain negative intrapleural pressure, it is necessary to closely monitor for pneumothorax and subcutaneous emphysema after chest tube clamping. Although the procedure was uneventful in our study, our protocol required that medical staff check each patient every 6 h after chest tube clamping. If abnormal symptoms developed, this interval would be shortened to 2–4 h after re-clamping, depending on the subjective symptoms of the patient and objective evaluation by the surgeon. In this study, there were no severe adverse events related to chest tube clamping. The rate of thoracocentesis after chest tube removal in the clamping group was comparable with that in the control group. A few patients had mild symptoms like dyspnea or pyrexia after clamping, but these were relieved after unclamping the chest tube in most cases. We have not reported the exact rates of such mild discomforts in this study, and this is a limitation that should be overcome in subsequent randomized clinical trials.
Two parameters influencing early chest tube removal following lobectomy have been investigated in clinical thoracic research studies: daily drainage amount and protein content of pleural drainage fluid. Several trials have used the amount of daily drainage as a criterion for chest tube removal. Cerfolio et al. (17) considered 450 mL/day of nonchylous drainage as the maximum amount of daily pleural drainage at which chest tube removal may be attempted. However, this is the most radical cutoff point recommended in the literature, and Grodzki (18) failed to validate this recommendation. Thus, a consensus on the threshold of drainage volume for safe chest tube removal has not yet been reached. Olgac et al. (6) suggested that the protein content of the pleural drainage fluid is a more reliable and precise criterion for chest tube removal than drainage amount, due to the poor absorption rate of proteins from the pleural surfaces. Therefore, the appropriate removal of chest tube requires two conditions: drainage volume below the safe threshold and protein density in the range of the absorption rate (otherwise, tube removal may cause excessive fluid accumulation). Taking these two factors into account, we designed the intermittent chest tube clamping protocol. It could test the feasibility of chest tube removal in the condition of clamping, which simulated the removal and converted back into draining conveniently. Protein-rich effusion fluid could be drained through the chest tube when the clamp was removed for half an hour in the morning. Our protocol is an option until a consensus is reached on the optimal chest tube removal protocol.
In the clamping group, the chest tube could not be removed in all cases in a short-time period. About 72.1% of the patients had duration of chest tube drainage less than or equal to 4 days in clamping group, while this proportion lowered to 50.8% in control group. It seems that the clamping protocol could make the clinical pathway more uniform and therefore standardize clinical procedure after lobectomy. For patients who still had high drainage volume after 4 days’ clamping, the decision to remove chest tube should be made more cautiously. Possible reasons include higher protein-rich content of the pleural drainage and impaired absorption rate of the pleural surfaces due to unknown causes.
In a multivariate logistic regression analysis, patients treated with VATS procedure experienced earlier chest tube removal. The mechanism may be attributed to the less invasive approach and decreased exudation from wound surface. Furthermore, clamping still played a role in shortening chest tube duration in this VATS subgroup (3.5±1.0 vs. 4.4±1.4 days, P=0.036). Thus, with the growing enthusiasm to adopt VATS approach as routine practice in the future, clamping protocol should furtherly enhance recovery by a possible synergistic effect with mini-invasive procedure in terms of reducing chest drainage.
A number of limitations in the present study must be considered. First, this was a retrospective cohort, with all its recognized limitations. Second, the application of propensity score matching shrank the sample size. It is necessary to investigate patient tolerance to the protocol more precisely by using a larger sample size. Third, historical comparisons between patients from different time periods may introduce bias into the study. As surgical techniques are continually updated, we could not exclude the possibility that the results were influenced by technological improvements. Therefore, further prospective investigation is warranted.
In conclusion, the findings of this study indicate that chest tube clamping may decrease the duration of chest tube drainage and shorten postoperative hospital stays without causing adverse effects.
Acknowledgements
We thank Elixigen Co. (Huntington Beach, CA, USA) for proofreading.
Funding: This work was supported by the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX201509) and the Beijing Municipal Science and Technology Commission (No. Z161100000516063).
Footnote
Conflicts of Interests: The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board of Beijing Cancer Hospital approved the study (ID: 2017KT27), and patient consent was waived.
References
- Czerny M, Fleck T, Salat A, et al. Sealing of the mediastinum with a local hemostyptic agent reduces chest tube duration after complete mediastinal lymph node dissection for stage I and II non-small cell lung carcinoma. Ann Thorac Surg 2004;77:1028-32. [Crossref] [PubMed]
- Pompili C, Brunelli A, Salati M, et al. Impact of the learning curve in the use of a novel electronic chest drainage system after pulmonary lobectomy: a case-matched analysis on the duration of chest tube usage. Interact Cardiovasc Thorac Surg 2011;13:490-3; discussion 493. [Crossref] [PubMed]
- Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg 2014;98:490-6; discussion 496-7. [Crossref] [PubMed]
- Alphonso N, Tan C, Utley M, et al. A prospective randomized controlled trial of suction versus non-suction to the under-water seal drains following lung resection. Eur J Cardiothorac Surg 2005;27:391-4. [Crossref] [PubMed]
- Russo L, Wiechmann RJ, Magovern JA, et al. Early chest tube removal after video-assisted thoracoscopic wedge resection of the lung. Ann Thorac Surg 1998;66:1751-4. [Crossref] [PubMed]
- Olgac G, Cosgun T, Vayvada M, et al. Low protein content of drainage fluid is a good predictor for earlier chest tube removal after lobectomy. Interact Cardiovasc Thorac Surg 2014;19:650-5. [Crossref] [PubMed]
- Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 2001;122:318-24. [Crossref] [PubMed]
- Utter GH. The rate of pleural fluid drainage as a criterion for the timing of chest tube removal: theoretical and practical considerations. Ann Thorac Surg 2013;96:2262-7. [Crossref] [PubMed]
- Brunelli A, Beretta E, Cassivi SD, et al. Consensus definitions to promote an evidence-based approach to management of the pleural space. A collaborative proposal by ESTS, AATS, STS, and GTSC. Eur J Cardiothorac Surg 2011;40:291-7. [Crossref] [PubMed]
- Gupta N. Pneumothorax: is chest tube clamp necessary before removal? Chest 2001;119:1292-3. [Crossref] [PubMed]
- Baumann MH, Strange C. The clinician's perspective on pneumothorax management. Chest 1997;112:822-8. [Crossref] [PubMed]
- Venuta F, Diso D, Anile M, et al. Chest Tubes: Generalities. Thorac Surg Clin 2017;27:1-5. [Crossref] [PubMed]
- Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 1997;10:219-25. [Crossref] [PubMed]
- Zocchi L. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 2002;20:1545-58. [Crossref] [PubMed]
- Negrini D, Ballard ST, Benoit JN. Contribution of lymphatic myogenic activity and respiratory movements to pleural lymph flow. J Appl Physiol (1985) 1994;76:2267-74. [Crossref] [PubMed]
- Negrini D, Pistolesi M, Miniati M, et al. Regional protein absorption rates from the pleural cavity in dogs. J Appl Physiol (1985) 1985;58:2062-7. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Results of a prospective algorithm to remove chest tubes after pulmonary resection with high output. J Thorac Cardiovasc Surg 2008;135:269-73. [Crossref] [PubMed]
- Grodzki T. Prospective algorithm to remove chest tubes after pulmonary resection with high output--is it valid everywhere? J Thorac Cardiovasc Surg 2008;136:536-author reply 536-7. [Crossref] [PubMed]



