Acute respiratory distress syndrome in traumatic brain injury: how do we manage it?
Introduction
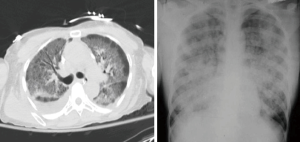
Acute respiratory distress syndrome (ARDS) is a life threatening condition characterized by refractory hypoxemia and stiff lungs (1-3). According to the recent Berlin Definition (4), ARDS is defined as an acute hypoxemic respiratory distress syndrome, not fully explained by cardiac failure occurring within one week of a known clinical insult or new or worsening respiratory symptoms, with bilateral opacities on chest X-ray (Table 1, Figure 1). A major component of ARDS is lung tissue inflammation.

Full table
In the Berlin Definition there is no more use of the term acute lung injury (ALI) and the wedge pressure measurement was abandoned because ARDS may coexist with hydrostatic oedema caused by cardiac failure or fluid overload, furthermore the value of using pulmonary artery catheterization was questioned due to insertion risk (4) (Table 2).

Full table
Several ventilatory strategies have been demonstrated to be useful in ARDS population, including the use of protective ventilation by using low tidal volume (TV) ventilation and limiting plateau pressure no more than 30 cmH2O with allowing permissive hypercapnia, prone positioning, the use of high positive end expiratory pressure (PEEP), recruitment manoeuvres (RM), extra corporeal membrane oxygenation (ECMO) and extra corporeal carbon dioxide removal (ECCO2R) (5-7).
The utility of these strategies has been proved in several groups of patients, both during anaesthesia and in critical care (8,9); however, their use in neurocritical care patients is still uncertain, as most of these lung protective ventilatory strategies are associated with an increased risk of intracranial hypertension (9).
There are tight interactions between cerebral and respiratory dynamics, so mechanical ventilation can have effect on cerebral perfusion and represent a potential burden for iatrogenic secondary brain damage (10). ARDS is common in neurocritical care patients (11-13) and lung injury is associated with worse outcome (12) and longer ICU length of stay (14).
Traumatic brain injury (TBI) is a major cause of mortality and morbidity and it is the most common cause of death under the age of 40 (15-17) (Figure 2).
According to the recently published Brain Trauma Foundation Guidelines, the main targets in TBI population are to avoid hypoxia and cerebral hypoperfusion (18). In particular, the central goal is the prevention of hypoxic secondary insults through the maintenance of an adequate cerebral perfusion pressure (CPP) and cerebral oxygen delivery.
Mechanical ventilation is very often necessary in the brain-injured patient and respiratory failure can be multi etiological [aspiration pneumonia, pulmonary contusion related to chest trauma, neurogenic pulmonary oedema, transfusion-related acute lung injury (TRALI) etc.].
When a concomitance of TBI and ARDS occurs, the ventilatory management can be very challenging as ventilatory targets are often in conflict in these two pathologies.
Recruitment maneuvers (RMs), prone positioning and the use of high PEEP can improve pulmonary gas exchange and respiratory mechanics by reducing ventilation—perfusion mismatch, and by opening collapsed alveoli reducing intrapulmonary shunt (19,20). However, they may be associated with the development of intracranial hypertension (21) by impairing jugular venous outflow and by impeding cerebral venous return to the right atrium. Moreover, they can increase ICP and decrease mean arterial pressure, both resulting in decreased CPP (22).
Literature is lacking regarding the management of patients with a concomitance of TBI and ARDS, and there is therefore need of a pragmatic approach to this group of patients.
The aim of this manuscript is to review and describe the different ventilatory strategies in patients with a concomitance of TBI and ARDS.
Ventilatory targets (Table 3)
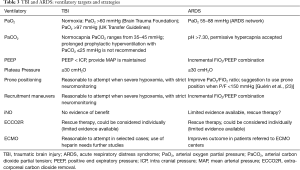
Full table
Arterial oxygen partial pressure (PaO2)
What really matters to the brain after TBI is to avoid hypoxemia, which has long been identified as a significant secondary insult following TBI and associated with poor outcome (24,25). PaO2 target can be efficiently directed on brain tissue oxygen tension (PbO2) or on jugular venous saturation (SjvO2 of <50%) (18). The effect on mortality and poor outcome of hypoxemia in TBI has been confirmed by the analysis of the IMPACT study database, a cohort of more than 9,000 patients with TBI recruited to randomized controlled trials and series dating back to the 1980s (26). The IMPACT analysis showed that arterial hypoxemia results in a decreased cerebral oxygen delivery, which causes cerebral vasodilatation, and an increase in ICP. A transcranial Doppler study in healthy volunteers found that the inflection point of cerebral vasodilatation is at PaO2 =58 mmHg or SpO2 of 90% (27). Current guidelines recommend avoidance of PaO2 <60 mmHg and maintenance of normoxia (18-22,24-28). The ARDSNet target of PaO2 is 55–80 mmHg seems therefore to be too low to be safely applied to patients with TBI (29).
PaCO2 and TV
The ARDSNet trial (29) demonstrated a decreased mortality and days of mechanical ventilation in patients with ARDS ventilated with TV of 6 mL/Kg compared to patients ventilated with 12 mL/kg (29). As expected, patients ventilated with lower TV had a higher mean PaCO2 than those in the traditional group (44 vs. 40 mmHg), and permissive hypercapnia as a consequence of protective ventilation is commonly accepted in patients with ARDS. However, hypercapnia is associated with cerebral vasodilation and consequent increased ICP, and can be dangerous in patients with TBI, and hypocapnia has been suggested to be a useful strategy to reduce ICP. According to the Brain Trauma Foundation Guidelines (18), prolonged prophylactic hyperventilation with PaCO2 of <25 mmHg is not recommended as first line therapy to reduce ICP, and hyperventilation should be avoided during the first 24 hours after injury when cerebral blood flow (CBF) is often critically reduced. Hyperventilation can be detrimental, as severe hypocapnia and consequent cerebral vasoconstriction can determine brain tissue hypoxia and compromise compliance and blood flow velocities (30,31); if hyperventilation is used, oxygen jugular saturation (SjO2) or brain tissue oxygen partial pressure (BtpO2) measurement are recommended to monitor oxygen delivery (IIB recommendation) (17). Grubb et al. (32) demonstrated that cerebral blood volume (CBV) is linearly related to PaCO2. Therefore, in TBI patients the standard of care is to ventilate to low normocapnia (17) (PaCO2 between 33.75 and 37.5 mmHg, equivalent to 4.5 to 5 KPa), but this may be a challenge in ARDS patients. Furthermore, high TV ventilation in patient with TBI has been associated with development of ARDS (14), as it has been shown that the proportion of induced ARDS increases with the higher initial TV, in particular with mean TV ≥10 mL/Kg (14).
All in, when ARDS and TBI coexist, a balance needs to be found between CO2 control and lung protection. Potentially, there are not absolute contraindications to the use of protective ventilation in TBI; the PaCO2 values should be set case by case according to ICP. Moreover, multimodal brain monitoring such as microdialysis catheters or brain parenchymal oxygen electrode may allow intensivists to tolerate a higher PaCO2, if cerebral metabolism remains intact.
Positive end-expiratory pressure
The use of PEEP has been considered very controversial in TBI patients, because the raised mean intrathoracic pressure related to PEEP can reduce cerebral venous return and consequently increase ICP (Figure 3). Cerebral perfusion during increased ICP is better described by a Starling Resistor (collapsible tube) than by Hagen-Poiseuille’ law (rigid tube). Accordingly, the water-fall principle best describes venous outflow; this means that cerebral venous downstream pressure is limited by CVP only when CVP exceeds ICP (the edge of the waterfall). If PEEP and CVP are lower than ICP, they don’t influence effective downstream pressure. Some authors (33) found that if PEEP values are below ICP values, then the associated augmentation of intrathoracic pressure doesn’t result in increased ICP. Observational studies found that high PEEP in patients with acute stroke and subarachnoidal haemorrhage (SAH) was associated with a reduced CPP and a decrease in CBF when cerebral autoregulation was impaired (34,35). However, they demonstrated that the principal mechanism responsible for reduction in CPP was a decrease in MAP, PEEP dependently. In all the cases, when MAP was restored, CPP and CBF returned to their baselines (35). Marcia et al. (36) demonstrated that when increased PEEP was applied to brain injured patients with ARDS, there was a substantial difference in the effects on ICP, depending on whether the application of PEEP caused alveolar hyperinflation or alveolar recruitment. When PEEP determines alveolar recruitment, the main effect is reduction in PaCO2 with subsequently reduction in ICP. The effect is opposite when PEEP causes alveolar hyperinflation (36).
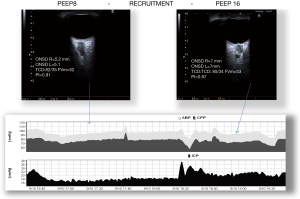
In a prospective study higher levels of PEEP were applied on patients with severe head injury or SAH with normal or low respiratory system compliance (37). In the group with normal respiratory compliance the increase of PEEP caused increased CVP, but reduced MAP, CPP and mean velocity of middle cerebral arteries. The authors concluded that monitoring respiratory system compliance may be useful to avoid negative effect of PEEP on ICP (37).
The optimal level of PEEP is still uncertain in ARDS patients. An ARDSnet study published in 2004 (38) found no benefit from higher PEEP strategy when compared with the standard ARDSnet ventilation protocol (39). At present, therefore, the use of PEEP to treat ARDS may be appropriate in TBI patients, provided that MAP is maintained and a strict close attention needs to be paid to any changes in CPP and ICP. When a decision of increasing PEEP in a TBI patient is made, it is necessary to ensure MAP stability and a close monitoring of cerebral parameters, mainly ICP and CPP.
RMs
RMs are useful strategies able to improve oxygenation, alveolar recruitment, and optimize ventilation-perfusion mismatch (1,20). However, RMs can have dangerous effect on ICP, as they can cause a significant elevation of ICP in patients with altered cerebral autoregulation, by impairing jugular blood outflow and increasing intrathoracic pressure, central venous pressure (CVP) and impeding cerebral venous return to the right atrium (Figure 3) (40).
Nemer et al. studied the effects of RMs in a RCT including patients with SAH and TBI who developed ARDS (40) and found that pressure control recruitment maneuver doesn’t impair ICP or CPP, while it improves oxygenation (40). This suggests that RMs can be used with caution in patients with TBI, ensuring hemodinamic stability and a close monitoring of cerebral parameters.
Prone positioning
Prone ventilation is known to improve PaO2/FiO2 ratio in ARDS (41). Recently published data from a large multicenter prospective randomised study (PROSEVA trial) showed a significant reduction of mortality after 28 and 90 days in ARDS treated with prone positioning compared to supine positioning (23). This recent meta-analysis by Guerin et al. (23) suggested use of prone position in ARDS patients with P/F <150 mmHg. These data followed a study conducted by Mancebo et al., which demonstrated a decrease in mortality from 58% to 43% with proning of patients for a more prolonged period (mean 17 hours) (42). In TBI patients there are serious concerns over the effect on ICP of prone positioning and also technical difficulties can be present, such as risks of removal or displacements of ICP Bolt and drains and practical difficulties in positioning neuromonitoring. The recommended position for patients with TBI is a 30 head up tilt combined with a straight head position (17). Therefore, this cohort of patients has previously been excluded in studies with utilization of prone position to improve oxygenation (43,44). Thelandersson et al. in their pilot study (45) demonstrated that prone positioning is not associated with adverse effect on ICP. In this study, the authors enrolled 12 patients mechanically ventilated and with an ICP probe inserted; the patients were placed in prone position for 3 hours and then turned them back to supine position. They demonstrated that positioning patients with TBI and reduced intracranial compliance in prone position significantly improved PaO2, SpO2 and respiratory system compliance, without altering intracranial parameters (45).
Nekludo et al. (46) discovered an improvement in oxygenation, a slightly increase of ICP and a moderate increase of MAP in TBI patients during treatment with prone position. As MAP increased to a greater extent than ICP, this resulted in an improved CPP in prone position. In their cohort of patients the authors also observed that the ARDS cases of extrapulmonary origin seem to respond better to the prone position, compared with pulmonary ARDS (46). In a more recent study conducted by Roth et al. (47) a moderate but significant elevation of ICP during prone positioning was demonstrated. However, the oxygenation during and after prone positioning shows a significant improvement and the achieved increase of oxygenation and PbO2 by far exceeds and the changes in ICP (47). In conclusion, there is no clear evidence to aid intensivists when deciding whether or not proning a patient when there is co-existence of ARDS and TBI; however, it doesn’t seem unreasonable to attempt prone ventilation when hypoxemia is refractory to conventional ventilation. The effect of proning on ICP should be observed in real continuous time, with additional treatments for increased ICP or deescalation to a different ventilatory strategy if ICP increases too much. We would not recommend proning in patients with frontal contusions, where the increased local pressure directly related to prone position may compromise the perfusion in the perilesional areas.
Nitric oxide (NO)
Inhaled NO has been proposed to treat refractory hypoxemia by reestablishing an adequate ventilation perfusion matching because of its pulmonary vasodilator effects. In both randomized clinical trials (48,49) and meta analyses (50-52), NO has been shown to improve oxygenation over a 24 hour period of treatment, but there have never been any convincing data on improving outcome and mortality. In addition, detrimental effects on kidney function have been documented (53). Papadimos et al. (54) demonstrated anti-inflammatory effects of inhaled nitric oxide beyond the respiratory system and hypothesized that it may be of benefit when TBI and ARDS coexist. However, the role of nitrix oxide after cerebral injury appears to be complex and linked with three different isoforms of enzyme NO syntase: iNO, eNO and nNO (55). In the acute phase nitrix oxide released from infiltrating leucocytes or produced by induced nitric oxide synthase contributes to secondary injury mechanisms, for example increasing the production of free radicals (56). Later, nitric oxide seems to have protective effects by improving CBF (56). The available evidence suggest that NO derives from eNOS is neuroprotective after brain injury, whereas NO synthesized by iNOS contributes to further damage.
Despite the controversies surrounding NO dysfunction after brain injury, there is animal evidence that increasing cerebral NO levels either directly using inhaled NO or indirectly using NO donors has neuroprotective effects (57). Better understanding of the role of NO pathway may lead to the development of new pharmacotherapies.
Extracorporeal membrane CO2 removal (ECCO2R)
The use of low TV (6 mLs/kg—predicted body weight) and maximum End Inspiratory Plateau Pressure of 30 cmH20 in ARDS patients (58), can determine hyperinflation and hypercapnia (59,60). Those results support the concept of ECCO2R as possible integrated tool to conventional ventilation to adjust respiratory acidosis (61). Although using an ECCO2R can provide a lower level of CO2 reached with less injurious ventilatory strategies, evidence from randomized control trials is lacking. The largest case series included 90 patients and demonstrated improvement in oxygenation and reduction of PaCO2 with the use of Arterio-Venous (AV) ECCO2R (62). There has been a small case series published describing the use of AV ECCO2R in five patients with TBI (63): in all of them PaO2/FiO2 ratio improved and PaCO2 decreased, and in some of them there was also a concomitant increase in ICP. The dose of anticoagulant required to run the extracorporeal circuit is lower than the one used for ECMO; however, there is still a significant risk of intracranial bleeding (63). In a pilot study of patients with TBI treated with ECCO2R (64), no complications (cerebral or extracerebral) attributable to anticoagulation were seen. Furthermore, this system can be run without additional heparin, as the components are heparin bonded, although the CO2 exchanger will need to be replaced more frequently (65). However, because of the small number of patients included in this analysis, larger prospective trials are warranted to further elucidate application of these devices in neurocritical care patients.
Extracorporeal membrane oxygenation (ECMO)
Veno-venous ECMO provides gas exchange across a semi permeable membrane and minimizes the trauma caused by mechanical ventilation allowing the lungs to rest (66,67). ECMO can be an effective strategy in patients with severe respiratory failure refractory to conventional ventilation (68,69). To avoid clotting of the circuit the patient needs to be anticoagulated with a bolus of heparin before cannulation (70) and then with heparin infusion to maintain ACT 180–200 s or PTT 40–50 s. Therefore, the main complication of ECMO is bleeding, which occurs in 17% to 21.3% of cases (71). Technical improvements of the ECMO devices, such as centifugal pump techniques and full heparin-coated circuits have led to a significant reduction of bleeding complications (72); however, ECMO is still considered contraindicated in patient with intracranial or active bleeding (73,74). There are several cases report published on successful craniotomy under ECMO treatment in multiple traumatized patients with severe thoracic and brain injuries, which reported good neurological outcomes (75,76). In the case report presented by Yen et al., the patient was not anticoagulated with systemic heparin for the running of the ECMO and a heparin bonded circuit was utilized (76). Robba et al. (77) presented a case series of four trauma patients managed with ECMO with no ECMO related complications. Mullenbach et al. (78) also recommended the use of heparin free ECMO in multiple injured patients with respiratory failure impossible to control by lung protective ventilation, even if severe TBI is present. Nowadays ECMO is feasible only in a limited number of centers, but despite the large number of complications associated with this treatment (bleeding, haemorrhage, acquired von-Willebrand disease during ECMO), it should be considered as rescue therapy for management of refractory respiratory failure even in trauma patients. Future larger studies should focus on the indications, management, and the use of heparine in this cohort of patients.
Steroids
The development and severity of ARDS are related to dysregulated inflammation and the outcomes are related to persistent inflammation and abnormal fibroproliferation (79,80). Corticosteroids are potent modulators of inflammation and inhibitors of fibrosis that have been used since the description of ARDS in attempts to improve outcome (81). Certainly there is no evidence to suggest that a short course of high dose steroids is helpful for either prevention, as demonstrated by four randomized controlled trials (82-85) or treatment of ARDS (86). Clinical trials regarding the use of steroids in the late stage of ARDS gave controversial results (87-89); therefore, work still needs to be done to determine if there are benefits to prolonged treatment with low dose corticosteroids for unresolving ARDS. However, ARDS is heterogeneous; steroids may be beneficial for some etiologies of ARDS, and not for others. ARDS associated with Pneumocystis carinii, for example, should be treated with steroids, since high quality randomized controlled trials have demonstrated that steroid treatment decreases both the risk of respiratory failure and death (90). Unfortunately, this level of evidence is lacking for most other etiologies of ARDS. Despite significant interest in the use of steroids in treatment of pandemic H1N1 influenza-associated ARDS, most reports from careful observation cohort studies suggest that such treatment was associated with harm (91,92). For TBI patients there has been a similar long interest in the use of steroids to modulate the disease process. However, now the use of steroids is not recommended for improving outcome or reducing ICP and in patients with severe TBI high dose methylprednisolone was associated with increased mortality and is contraindicated. (Level 1 Recommendation, Guidelines for the Management of Severe TBI, 4th edition) (17). The Corticosteroid Randomization after Significant Head Injury Trial (CRASH) (93) was designed to provide high quality of evidence on the impact of steroids on TBI patients. This was a large multicenter trialwhich studied over 10,000 patients with TBI. Participants were randomized to receive either 2 g intravenous methylprednisolone followed by 0.4 mg/h for 48 h, or placebo. Data from CRASH study showed a deleterious effect of methylprednisolone, higher mortality and more corticosteroid-treated subjects in the unfavorable outcomes group (death and severe disability) compared with the favorable group (94).
Fluid balance and hemoglobin target
An association between positive fluid balance and worse outcome in patients with ARDS has been demonstrated in a number of studies (95,96). Data from the ARDS Network Fluids and Catheter Treatment Trial (97) support the use of a conservative fluid management strategy in ARDS, having demonstrated improvement of the oxygenation index and the lung injury score, reduction of ventilator-free days and length of stay in ICU. However, in some patients, particularly those with severe ARDS requiring high mean airway pressures for oxygenation, hypovolaemia may exacerbate hypoxaemia by virtue of increased intrapulmonary shunt, and clinical benefit may result from the careful administration of fluid boluses (98). Zhao et al. (99) were the first to test the effect of fluid balance on short term TBI outcome and they demonstrated that both high and low fluid balances were associated with poor short-term outcome and unstable ICP in TBI patients. Patients at the low (<637 mL fluid balance calculated at midnight) and upper (>3,673 mL calculated at midnight) tertiles of fluid balance were associated with poor outcomes. Those in the upper tertile also had a higher incidence of acute kidney injury and refractory intracranial hypertension. There was a negative correlation between the cumulative fluid balance and the short-term outcome for patients in the low tertile and a positive correlation between the cumulative fluid balance and the short-term outcome in the upper fluid balance group (99). High fluid balance is independently associated with poor short-term outcomes including acute kidney injury and refractory intracranial hypertension in patients with TBI. An insufficient fluid in the early stage of critical illness may lead to tissue hypoperfusion and ischemia (100) whereas excessive intravenous fluid contributes to the development of tissue edema. An optimal volume of fluid at any given time maintains tissue viability (101). These data suggest the critical importance of maintaining an appropriate fluid balance for TBI patients. Excessive fluid balance may exacerbate secondary brain injures such as edema, intracranial hypertension and the disruption of the blood-brain barrier, leading to worse outcome. However, fluid therapy is necessary for volume resuscitation, maintenance of CPP and the prevention of secondary brain injury (102). Anemia is highly prevalent in the intensive care unit (ICU) with up to 95% critically ill patients developing subnormal haemoglobin (Hb) level by day 3 (103). Likewise, 20% to 53% of patients receive red blood cell (RBC) transfusions to correct anemia during their ICU stay (104). However, allogenic RBC transfusions carry risks that may adversely affect clinical outcomes (105,106). Evidence suggests that it is safe to adopt a lower transfusion threshold for the general medical/surgical ICU population (107-109). This has led to a paradigm shift concerning RBC transfusions in the ICU, with most guidelines now recommending hemoglobin levels around 70 g/L for transfusion in patients without significant comorbidities to minimize exposure to allogenic blood (110-112). However, specific patient populations, such as neurocritically ill patients, were underrepresented in these studies and results could thus not be applied to them. Neurocritically ill patients may represent an exception to the rationale for using low transfusion triggers because impaired oxygen delivery is a crucial modifiable factor in brain ischemia and secondary brain injury (113,114). The optimal Hb level for cerebral oxygen delivery in TBI patients is still unknown (115). Two guidelines in neurocritically ill patient population (subarachnoid hemorrhage) were recently published; one recommending to treat anemia but without mention to threshold, and the other one recommending transfusion in order to reach hemoglobin levels of 80 to 100 g/L (116,117). Interestingly, guidelines for the management of patients with TBI did not cover this topic (18). Moreover, data on which clinicians have to rely in decision making is discordant, as both anemia and RBC transfusion have been observed to be associated with unfavorable clinical outcomes in neurocritically ill patients (118,119). Anemia has repeatedly shown to be associated with unfavorable outcomes in patients with TBI (118-120), although other studies have not confirmed this relationship (121,122). Recent microdialysis studies showed that cerebral metabolism of subjects affected by SAH and TBI becomes impaired at Hb values lower than 90 g/L (123), but RBC transfusions are known to improve physiologic measures such as brain oxygen tension in a majority of patients with TBI (124-126).
Conclusions
The lung and brain interaction poses important challenges to ventilator management of patients with TBI and ARDS. The beneficial effect of protective lung ventilation and respiratory strategies is well established both in intensive care and in the operating room. However, the application of these techniques on neurocritical care patients is contrasting because of the specific needs and ventilator targets in this group of patients. Moreover, haemodynamic and general management (including Hb target and fluid balance) can be contrasting in this group of patients. The use of cerebral multimodal monitoring can be useful to assess cerebral hemodynamic and to evaluate the effects of ventilator strategies commonly used in ARDS patients. It would be desiderable a concomitant cerebral and respiratory system monitoring in these patients, mainly in the more severe ones, in order to allow the best approach to these patients and avoid complications.
Further studies will be necessary to create shared diagnostic and therapeutic guidelines based on available evidence and that can contribute to improve patients’ clinical outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49. [Crossref] [PubMed]
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967;2:319-23. [Crossref] [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med 1994;20:225-32. [Crossref] [PubMed]
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute Respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Fanelli V, Vlachou A, Ghannadian S, et al. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis 2013;5:326-34. [PubMed]
- Ranieri VM, Giunta F, Suter PM, et al. Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA 2000;284:43-4. [Crossref] [PubMed]
- Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005;33:1-6. [Crossref] [PubMed]
- Fernandez-Bustamante A, Hashimoto S, Neto AS, et al. Perioperative Lung Protective Ventilation in obese patients. BMC Anesthesiol 2015;15:56-65. [Crossref] [PubMed]
- Borsellino B, Schultz MJ, Gama de Abreu M, et al. Mechanical ventilation in neurocritical care patients: a systematic literature review. Expert Rev Respir Med 2016;10:1123-32. [Crossref] [PubMed]
- Nyquist P, Stevens RD, Mirski MA. Neurologic injury and mechanical ventilation. Neurocrit Care 2008;9:400-8. [Crossref] [PubMed]
- Bratton SL, Davis RL, et al. Acute lung injury in isolated traumatic brain injury. Neurosurgery 1997;40:707-12. [Crossref] [PubMed]
- Holland MC, Mackersie RC, Morabito D, et al. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma 2003;55:106-11. [Crossref] [PubMed]
- Kahn JM, Caldwell EC, Deem S, et al. Acute lung injury in patients with Subarachnoid Hemorrhage: incidence, risk factors and outcome. Crit Care Med 2006;34:196-202. [Crossref] [PubMed]
- Mascia L, Zavala E, Bosma K, et al. High tidal Volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med 2007;35:1815-20. [Crossref] [PubMed]
- Menon DK, Schwab K, Wright DW, et al. Position Statement: Definition of Traumatic Brain Injury. Arch Phys Med Rehabil 2010;91:1637-40. [Crossref] [PubMed]
- National Institute for Health and Clinical Excellence: Guidance. Head injury: triage, assessment, investigation and early management of head injury in children, young people and adults. London: National Institute for Health and Care Excellence (UK), 2014.
- Lawrence T, Helmy A, Boumra O, et al. Traumatic brain injury in England and Wales: prospective audit of epidemiology, complications and standardised mortality. BMJ Open 2016;6:e012197. [Crossref] [PubMed]
- Carney N, Totten AM, O'Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017;80:6-15.
- Sud S, Friedrich JO, Adhikari NK, et al. Effect of prone positioning during mechanical ventilation on mortality among patients with acute respiratory distress syndrome: A systematic review and meta-analysis. CMAJ 2014;186:E381-90. [Crossref] [PubMed]
- Gattinoni L, Pelosi P, Crotti S, et al. Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med 1995;151:1807-14. [Crossref] [PubMed]
- Robba C, Bragazzi NL, Bertuccio A, et al. Effects of prone position and positive end- expiratory pressure on noninvasive estimators of ICP: a pilot study. J Neurosurg Anesthesiol 2017;29:243-50. [Crossref] [PubMed]
- Georgiadis D, Schwarz S, Baumgartner RW, et al. Influence of positive end expiratory pressure on intracranial pressure and cerebral perfusion pressure in patients with acute stroke. Stroke 2001;32:2088-92. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Jones PA, Andrews PJ, Midgley S, et al. Measuring the burden of secondary insults in head-injured patients during intensive care. J Neurosurg Anesthesiol 1994;6:4-14. [Crossref] [PubMed]
- Wald SL, Shacford SR, Fenwick J. The effect of secondary insults on mortality and long term disability after severe head injury in a rural region without a trauma system. J Trauma 1993;34:377-81. [Crossref] [PubMed]
- McHugh GS, Engel DC, Butcher I, et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma 2007;24:287-93. [Crossref] [PubMed]
- Gupta AK, Menon DK, Czosnyka M, et al. Thresholds for hypoxic cerebral vasodilatation in volunteers. Anesth Analg 1997;85:817-20. [Crossref] [PubMed]
- The Association of Anaesthetists of Great Britain and Ireland, Recommendations for the safe transfer of patients with brain injury; 2006. Available online: http://www.aagbi.org/publications/guidelines/docs/braininjury.pdf
- The Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Teismann IK, Oeschlager C, Wrestler N, et al. Discontinuous versus continuous weaning in stroke patients. Cerebrovasc Dis 2015;39:269-77. [Crossref] [PubMed]
- Bilotta F, Robba C, Santoro A, et al. Contrast-enhanced ultrasound imaging in detection of changes in cerebral perfusion. Ultrasound Med Biol 2016;42:2708-16. [Crossref] [PubMed]
- Grubb RL, Raichie ME, Eichling JO, et al. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke 1974;5:630-9. [Crossref] [PubMed]
- McGuire G, Crossley D, Richards J, et al. Effects of varying levels of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure. Crit Care Med 1997;25:1059-62. [Crossref] [PubMed]
- Georgiadis D, Schwarz S, Baumgartner RW, et al. Influence of positive end expiratory pressure on intracranial pressure and cerebral perfusion pressure in patients with acute stroke. Stroke 2001;32:2088-92. [Crossref] [PubMed]
- McGuire G, Bauhuf C, Roth H, et al. Effects of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure. Crit Care Med 1997;25:1059-62. [Crossref] [PubMed]
- Mascia L, Grasso S, Fiore T, et al. Cerebro-pulmonary interactions during the application of low levels of positive end expiratory pressure. Intensive Care Med 2005;31:373-9. [Crossref] [PubMed]
- Caricato A, Conti G, Della Corte F, et al. Effects of PEEP on the intracranial system of patients with head injury and subarachnoid hemorrage: the role of respiratory system compliance. J Trauma 2005;58:571-6. [Crossref] [PubMed]
- Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end expiratory pressure in patients with acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. [Crossref] [PubMed]
- ARDSnet ventilation protocol summary; 2008. Available online: http://www.ardsnet.org/
- Nemer SN, Caldeira JB, Azeredo LM, et al. Alveolar recruitment maneuver in patients with subarchnoid hemorrhage and acute respiratory distress syndrome: a comparison of two approaches. J Crit Care 2011;26:22-7. [Crossref] [PubMed]
- Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001;345:568-73. [Crossref] [PubMed]
- Mancebo J, Fernandez R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;173:1233-9. [Crossref] [PubMed]
- Blanch L, Mancebo J, Perez M, et al. Short term effects of prone position in critically ill patients with acute respiratory distress syndrome. Intensive Care Med 1997;23:1033-9. [Crossref] [PubMed]
- Johannigman JA, Davis K, Miller S, et al. Prone position for acute respiratory distress syndrome in the surgical intensive care unit: who, when, and how long? Surgery 2000;128:708-16. [Crossref] [PubMed]
- Thelandersson A, Cider A, Nellgard B. Prone position in mechanically ventilated patients with reduced intracranial compliance. Acta Anaesthesiol Scand 2006;50:937-41. [Crossref] [PubMed]
- Nekludov M, Bellander BM, Mure M. Oxygenation and cerebral perfusion pressure improved in the prone position. Acta Anaesthesiol Scand 2006;50:932-6. [Crossref] [PubMed]
- Roth C, Ferbert A, Deinsberger W, et al. Does Prone Positioning Increase Intracranial Pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care 2014;21:186-91. [Crossref] [PubMed]
- Taylor RW, Zimmerman JL, Dellinger RP, et al. Low dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA 2004;291:1603-09. [Crossref] [PubMed]
- Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute distress respiratory syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med 1998;26:15-23. [Crossref] [PubMed]
- Karam O, Gebostorf F, Wetterslev J, et al. The effect of inhaled nitric oxide in acute respiratory distress syndrome in children and adults: a Cochrane systematic review with trial sequential analysis. Anaesthesia 2017;72:106-17. [Crossref] [PubMed]
- Adhikari NK, Burns KE, Friedrich JO, et al. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta analysis. BMJ 2007;334:779-88. [Crossref] [PubMed]
- Afshari A, Brok J, Moller AM, et al. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. Cochrane Database Syst Rev 2010.CD002787. [PubMed]
- Ruan SY, Wu HY, Lyn HH, et al. Inhaled nitric oxide and the risk of renal dysfunction in patients with acute respiratory distress syndrome: a propensity match chort study. Crit Care 2016;20:389. [Crossref] [PubMed]
- Papadimos TJ. The beneficial effect of inhaled nitric oxide in patients with severe traumatic brain injury complicated by acute respiratory syndrome: a hypothesis. J Trauma Manag Outcomes 2008;2:1. [Crossref] [PubMed]
- Toda N, Ayajiiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev 2009;61:62-97. [Crossref] [PubMed]
- Cherian L, Hlatki R, Robertson CS. Nitric oxide in traumatic brain injury. Brain Pathol 2004;14:195-201. [Crossref] [PubMed]
- Guo ZN, Shao A, Tong LS, et al. The role of Nitric Oxide and Sympathetic control in cerebral autoregulation in the setting of Subarachnoid Haemorrage and traumatic brain injury. Mol Neurobiol 2016;53:3606-15. [Crossref] [PubMed]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2007;175:160-6. [Crossref] [PubMed]
- Hager DN, Krishnan JA, Hayden DL, et al. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med 2005;172:1241-5. [Crossref] [PubMed]
- Terragni PP, Del Sorbo L, Mascia L, et al. Tidal volume lower than 6ml/Kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology 2009;111:826-35. [Crossref] [PubMed]
- Bein T, Weber F, Philipp A, et al. A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med 2006;34:1372-7. [Crossref] [PubMed]
- Bein T, Scherer MN, Philipp A, et al. Pumpless extracorporeal lung assist (pECLA) in patients with acute respiratory distress syndrome and severe brain injury. J Trauma 2005;58:1294-7. [Crossref] [PubMed]
- Munoz-Bendix C, Beseoglu K, Kram R. Extracorporeal decarboxilation in patients with severe traumatic brain injury and ARDS enables effective control of intracranial pressure. Critical Care 2015;19:381-7. [Crossref] [PubMed]
- Kreyer S, Muders T, Luepschen H, et al. The effect of pumpless extracorporeal CO2 removal on regional perfusion of the brain in experimental acute lung injury. J Neurosurg Anesthesiol 2013;25:324-9. [Crossref] [PubMed]
- Schuerer DJ, Kolovos NS, Boyd KV, et al. Extracorporeal membrane oxygenation: current clinical practice, coding, and reimbursement. Chest 2008;134:179-84. [Crossref] [PubMed]
- Chalwin RP, Moran JL, Graham PL. The role of extracorporeal membrane oxygenation for treatment of the adult respiratory distress syndrome: review and quantitative analysis. Anaesth Intensive Care 2008;36:152-61. [PubMed]
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized propspective study. JAMA 1979;242:2193-6. [Crossref] [PubMed]
- Hung M, Vuylsteke A, Valchanov K. Extracorporeal membrane oxygenation: coming to an ICU near you. Journal of the Intensive Care Society 2012;13:241-52. [Crossref]
- Biswas AK, Lewis L, Sommerauer JF. Aprotinin in the management of life-threatening bleeding during extracorporeal life support. Perfusion 2000;15:211-16. [Crossref] [PubMed]
- Extracorporeal Life Support Organization. ECMO Registry Report of the ELSO: International Summary. Michigan: ELSO; 2011.
- Madershahian N, Wittwer T, Strauch J, et al. Application of ECMO in multitrauma patients with ARDS as a rescue therapy. J Card Surg 2007;22:180-4. [Crossref] [PubMed]
- Cordell-Smith JA, Roberts N, Peek GJ, et al. Traumatic lung injury treated by extracorporeal membrane oxygenation (ECMO). Injury 2006;37:29-32. [Crossref] [PubMed]
- Arlt M, Philipp A, Voelkel S, et al. Extracorporeal membrane oxygenation in severe trauma patients with bleeding shock. Resuscitation 2010;81:804-9. [Crossref] [PubMed]
- Friesenecker BE, Peer R, Rieder J, et al. Craniotomy during ECMO in a severely traumatized patient. Acta Neurochir (Wien) 2005;147:993-6. [Crossref] [PubMed]
- Yen TS, Liau CC, et al. Extracorporeal membrane oxygenation resuscitation for traumatic brain injury after decompressive craniotomy. Clin Neurol Neurosurg 2008;110:295-7. [Crossref] [PubMed]
- Robba C, Ortu A, Lombardo A, et al. Extra Corporeal Membrane Oxygenation is an effective rescue treatment for acute respiratory distress syndrome in trauma patients: a case series and systematic review of literature. J Trauma Acute Care Surg 2017;82:165-73. [Crossref] [PubMed]
- Muellenbach RM, Kredel M, Kunze E, et al. Prolonged heparin free extracorporeal membrane oxygenation in multiple injured acute respiratory distress syndrome patients with Traumatic Brain Injury. J Trauma Acute Care Surg 2012;72:1444-7. [Crossref] [PubMed]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49. [Crossref] [PubMed]
- Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 2011;6:147-63. [Crossref] [PubMed]
- Martin C, Papazian L, Payan MJ, et al. Pulmonary fibrosis corelates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest 1995;107:196-200. [Crossref] [PubMed]
- Bone RC, Fisher CJ Jr, Clemmer TP, et al. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest 1987;92:1032-6. [Crossref] [PubMed]
- Weigelt JA, Norcross JF, Borman KR, et al. Early steroid therapy for respiratory failure. Archives of surgery 1985;120:536-40. [Crossref] [PubMed]
- Schein RM, Bergman R, Marcial EH, et al. Complement activation and corticosteroid therapy in the development of the adult respiratory distress syndrome. Chest 1987;91:850-4. [Crossref] [PubMed]
- Luce JM, Montgomery AB, Marks JD, et al. Ineffectiveness of high- dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis 1988;138:62-8. [Crossref] [PubMed]
- Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002;288:862-71. [Crossref] [PubMed]
- Hooper RG, Kearl RA. Established adult respiratory distress syndrome succesfully treated with corticosteroids. South Med J 1996;89:359-64. [Crossref] [PubMed]
- Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome. JAMA 1998;280:159-65. [Crossref] [PubMed]
- Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory syndrome. N Engl J Med 2006;354:1671-84. [Crossref] [PubMed]
- Gagnon S, Boota AM, Fischl MA, et al. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: A double-blind, placebo-controlled trial. N Engl J Med 1990;323:1444-50. [Crossref] [PubMed]
- Martin-Loeches I, Lisboa T, Rhodes A, et al. Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. Intensive Care Med 2011;37:272-83. [Crossref] [PubMed]
- Brun-Buisson C, Richard JC, Mercat A, et al. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress síndrome. Am J Respir Crit Care Med 2011;183:1200-6. [Crossref] [PubMed]
- Roberts I, Yates D, Sandercock P, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet 2004;364:1321-8. [Crossref] [PubMed]
- Edwards P, Arango M, Balica L, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroids in adults with head injury-outcomes at 6months. Lancet 2005;365:1957-9. [Crossref] [PubMed]
- Sakr Y, Vincent JL, Reinhart K, et al. Sepsis Occurrence in Acutely Ill Patients I: High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest 2005;128:3098-108. [Crossref] [PubMed]
- Grams ME, Estrella MM, Coresh J, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network: Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol 2011;6:966-73. [Crossref] [PubMed]
- Wiedemann HP, Wheeler AP, Bernard GR, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564-75. [PubMed]
- Gattinoni L, Cressoni M, Brazzi L. Fluids in ARDS: from onset to recovery. Curr Opin Crit Care 2014;20:373-7. [Crossref] [PubMed]
- Zhao Z, Wang D, Jia Y, et al. Analysis of the association of fluid balance and short-term outcome in traumatic brain injury. J Neurol Sci 2016;364:12-8. [Crossref] [PubMed]
- Murugan R, Kellum JA. Fluid balance and outcome in acute kidney injury: is fluid really the best medicine? Crit Care Med 2012;40:1970-2. [Crossref] [PubMed]
- Gantner D, Moore EM, Cooper DJ. Intravenous fluids in traumatic brain injury: what's the solution? Curr Opin Crit Care 2014;20:385-9. [Crossref] [PubMed]
- Kahle KT, Walcott BP, Simard JM. Continuous hyperosmolar therapy for traumatic brain injury-associated cerebral edema: as good as it gets, or an iatrogenic secondary insult? J Clin Neurosci 2013;20:30-1. [Crossref] [PubMed]
- Corwin HL, Gettinger A, Pearl RG, et al. The CRIT Study: anemia and blood transfusion in the critically ill: current clinical practice in the United States. Crit Care Med 2004;32:39-52. [Crossref] [PubMed]
- Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA 2002;288:1499-507. [Crossref] [PubMed]
- Rawn J. The silent risks of blood transfusion. Curr Opin Anaesthesiol 2008;21:664-8. [Crossref] [PubMed]
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care: Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409-17. [Crossref] [PubMed]
- Lacroix J, Hébert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007;356:1609-19. [Crossref] [PubMed]
- Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med 2008;36:2667-74. [Crossref] [PubMed]
- Carless PA, Henry DA, Carson JL, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 2010.CD002042. [PubMed]
- Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of red blood cells. Blood Transfus 2009;7:49-64. [PubMed]
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008;36:296-327. [Crossref] [PubMed]
- American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology 2006;105:198-208. [Crossref] [PubMed]
- Spiotta AM, Stiefel MF, Gracias VH, et al. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J Neurosurg 2010;113:571-80. [Crossref] [PubMed]
- Narotam PK, Morrison JF, Nathoo N. Brain tissue oxygen monitoring in traumatic brain injury and major trauma: outcome analysis of a brain tissue oxygen-directed therapy. J Neurosurg 2009;111:672-82. [Crossref] [PubMed]
- Pendem S, Rana S, Manno EM, et al. A review of red cell transfusion in the neurological intensive care unit. Neurocrit Care 2006;4:63-7. [Crossref] [PubMed]
- Diringer MN, Bleck TP, Claude Hemphill J 3rd, et al. Neurocritical Care Society: Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the neurocritical care Society’s multidisciplinary consensus conference. Neurocrit Care 2011;15:211-40. [Crossref] [PubMed]
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012;43:1711-37. [Crossref] [PubMed]
- Utter GH, Shahlaie K, Zwienenberg-Lee M, et al. Anemia in the setting of traumatic brain injury: the arguments for and against liberal transfusion. J Neurotrauma 2011;28:155-65. [Crossref] [PubMed]
- Kramer AH, Zygun DA. Anemia and red blood cell transfusion in neurocritical care. Crit Care 2009;13:R89. [Crossref] [PubMed]
- Leal-Noval SR, Munoz-Gomez M, Murillo-Cabezas F. Optimal hemoglobin concentration in patients with subarachnoid hemorrhage, acute ischemic stroke and traumatic brain injury. Curr Opin Crit Care 2008;14:156-62. [Crossref] [PubMed]
- Carlson AP, Schermer CR, Lu SW. Retrospective evaluation of anemia and transfusion in traumatic brain injury. J Trauma 2006;61:567-71. [Crossref] [PubMed]
- Schirmer-Mikalsen K, Vik A, Gisvold SE, et al. Severe head injury: control of physiological variables, organ failure and complications in the intensive care unit. Acta Anaesthesiol Scand 2007;51:1194-201. [PubMed]
- Kurtz P, Schmidt JM, Claassen J, et al. Anemia is associated with metabolic distress and brain tissue hypoxia after subarachnoid hemorrhage. Neurocrit Care 2010;13:10-6. [Crossref] [PubMed]
- Figaji AA, Zwane E, Kogels M, et al. The effect of blood transfusion on brain oxygenation in children with severe traumatic brain injury. Pediatr Crit Care Med 2010;11:325-31. [PubMed]
- Smith MJ, Stiefel MF, Magge S, et al. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med 2005;33:1104-8. [Crossref] [PubMed]
- Leal-Noval SR, Rincon-Ferrari MD, Marin-Niebla A, et al. Transfusion of erythrocyte concentrates produces a variable increment on cerebral oxygenation in patients with severe traumatic brain injury: a preliminary study. Intensive Care Med 2006;32:1733-40. [Crossref] [PubMed]