Posterior uniportal video-assisted thoracoscopic surgery for anatomical lung resections
Introduction
Since it was described for the first time in 2011 (1) uniportal video-assisted thoracoscopic surgery (uVATS) for anatomical lung resection has grown in use worldwide.
uVATS has been utilized for a wide spectrum of thoracic interventions and is now used for almost all the indications as in open surgery (2-4).
Typically uVATS begins with a 3–5 cm incision in 4th or 5th intercostal space on the anterior axillary line, regardless of the type (anterior or posterior) of resection which should be undertaken.
Posterior lung segments, like apical segments of both lower lobes and posterior segments of both upper lobes, might be more easily accessible in a uniportal fashion from behind, making an incision in the “triangle of auscultation” (5-7).
The purpose of this study is to present a case-series of this novel approach for uVATS predominantly used for posterior segmentectomies, and if needed, also for lobectomies.
Methods
An observational retrospective audit study which included the first 20 patients who underwent anatomical resection by a single surgeon utilizing the posterior uVATS (puVATS) method from January 2016–June 2017 was undertaken.
Surgical technique
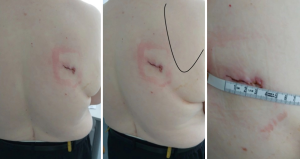
A single incision of 3.5–4.5 cm was made in the 6th intercostal space, between the latissimus dorsi and trapezius muscles, in the so-called ‘triangle of auscultation’ (Figures 1-3) (6). Both surgeon and assistant are positioned on the back of the patient, while scrub nurse approaches from the opposite side. The monitors are located on both sides of the patient.
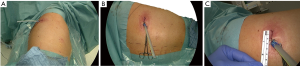
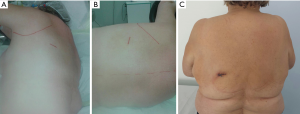
In most of the cases it was not necessary to dissect in the fissure, but to staple it either in the beginning (Figure 4), or at the end of resection (9).

Superior segment of the right lower lobe
Bronchial tree was initially assessed, dissecting along the bronchus intermedius and exposing the apical segment bronchus. After the division of the apical segment bronchus, the segment vein and artery were easily exposed. Each vascular structure was separately secured and divided with a stapler. Dissection of the fissure was not required as the segment was detached from the rest of the lung parenchyma using multiple staplers along the potential segmental fissure line.
In cases which required lobectomy (9) the lower lobar vein was exposed and detached, followed by the exposure of the bronchus, both for lower and middle lobes. After the division of the lower lobe bronchus, the artery for basilar segments was easily exposed with close attention paid to the location of the middle lobe artery. Finally, the fissure between the lower and middle lobes was stapled as required and the lower lobe was detached, bagged and removed.
Posterior segment of the right upper lobe (9)
Bronchial bifurcation was immediately approached. From that point, it was possible either to access to segmental bronchus or to undermine the fissure, divide it and expose the artery for this segment. In the first case, after the division of the segmental bronchus, segmental artery emerged immediately behind it, while the vein appeared behind the artery. In the second case, after the removal of local lymph nodes (station 11), segment artery was assessed and divided. The vein for the posterior segment of the right upper lobe was then approached and divided. Following the clamping of the segmental bronchus, bronchoscopy was performed and the bronchus was divided. Posterior segment of the right upper lobe was then detached from the rest of the lung parenchyma, using multiple staplers along the potential segmental fissure line.
Superior segment of the left lower lobe (10)
This procedure doesn’t differ from the right as detailed above. The first bronchus branch on the left lower bronchus was the one for the apical segment. Behind, or sometimes beside it the artery for the same segment was laid. Sometimes there were two arteries. The vein going out of this segment was more often laid behind the segmental bronchus and was approachable after detaching of the bronchus. Sometimes this vein could be easily identified in the hilum joining the left lower vein rather distally, shortly before the vein entered the pericardium and in this case this segmental vein could be detached early in the operation. At the end, like on the right lung, parenchyma was detached and the segment was removed.
If lobectomy was required, the lower lobe vein was easily approached and divided, followed by the dissection and division of the left lower lobe bronchus. The artery for the basal segment group was than to be divided. After the local lymph node dissection, the lower lobe was detached from the upper along the fissure.
Posterior segment of the left upper lobe
After dissection of the hilum, the posterior segmental artery was easily identified. This was, however, not always the case with the segmental bronchus as it was immediately behind the artery surrounded with the lingular and the apical segmental artery, as well as the main stem of the artery. Careful dissection was required. After encircling the bronchus, bronchoscopic confirmation was mandatory, as inflation of the whole lung could be misleading, leading at the same time to inflation of the whole upper lobe without strict demarcation of this segment. The vein of this segment was difficult to locate, however, the vein was resected along with the stapled parenchyma. Without strict demarcation lines, the exact margins of the segment could not be precisely established and therefore it was up to the surgeon to determine the optimal line of stapling for resection.
If lobectomy was warranted, which happened to be the case twice in our series, the fissure was opened from behind, after a tunnel above the artery was created. That approach allowed access to the lingula arter yor arteries, as well as the lymphatic sump. After completion of the fissure and division of the segmental apical artery, the next structure could be either the anterior segmental artery or the lobar bronchus, depending on the anatomical appearance. At the end, a final stapling was placed on the upper lobe vein and the lobe was detached.
After the resection, a single 24-F chest tube (Rocket Medical, Washington, DC, USA) was placed through the same incision and connected to the suction device (Thopaz Drainage System, Medela, Switzerland). The chest tube was removed after cessation of the air leak or after daily drainage was under 5 mL/kg of the patient.
At the end of the procedure, intercostal nerve block has been performed to all of the patients by instillation 20 mL 1% prilocaine hydrochlorid. Level of pain was assessed twice daily according to the visual-analog scale (VAS), under standardized pain management.
Our standardized therapy consists of one non-steroid anti-inflammatory drug (NSAID), typically metamizol (4 g daily) and one opioid agent—oxycodone hydrochlorid (10 mg/5 mg BID). This regime was expanded by a higher dosage of oxycodone if needed. As soon as a thoracic drainage was removed, the opioid agent was omitted as well. However, in case of persistent pain, the same opioid agent was retained. Overall oxycodone consumption was measured.
Informed consent was obtained from the patients for publication of this manuscript and any accompanying images. Ethical Committee on human research of the Heidelberg Medical School approved the retrospective evaluation of the data in anonymous fashion (S-430/2017).
Results
Thirteen women and seven men with a median age of 66 (range, 44–81) years were included in this cohort.
Two patients had metastatic disease while 18 had a primary lung tumor, one of which was benign. There were 10 adenocarcinoma (two lepidic) of the lung, four squamous cell lung carcinoma, three carcinoid tumors (one atypic) and one 2 cm large hamartoma.
This cohort underwent 5 posterior and 3 apical segmentectomies of the right upper lobe and 6 apical segmentectomies of the left lower lobe. Moreover, there were 6 lobectomies, all except for one as an extension of initially planned “posterior” segmentectomy (Table 1).
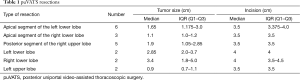
Full table
Conversion to thoracotomy or an additional port placement was not required in any patient. There were no intraoperative complications. Median operative time (IQR) was 160 minutes (142–178 minutes). Median number of removed lymph nodes (IQR) was 19 [15–20]. Median BMI was 29 (range, 20–45), median ASA score was 3, while age adjusted Charlson’s comorbidity index (ACCI) score was 10 (range, 4–17). Four patients had preoperative chemotherapy, two due to a previous carcinoma (hypopharynx and colon), one due to a small cell lung cancer 12 years ago treated with a combined radio-chemotherapy (inclusive irradiation of the CNS for a metastasis) and one after contralateral lobectomy for a lung cancer 5 years before the present diagnosis.
Average days with a chest tube was 3 (range, 2–4). There was no prolonged air leak, moreover only one patients showed air leak which spontaneously ceased on postoperative day (POD) 3.
Median postoperative pain (IQR) on the POD 1 was 4 [3–6], on the POD 3 was 2 [1–3] and on the day of discharge was 1 [0.5–1]. Median consumption of the oxycodone (IQR) was 50 mg (40–80 mg). One patient was dropped out from pain evaluation due to a chronicle pain syndrome treated with opioids.
Four patients had postoperative complications which included bronchitis in three and heart failure in one. All recovered and were discharged. Median length of hospital stay (IQR) was 6 days (5–8 days).
Discussion
The posterior approach for the anatomical resection of the posterior lung segments appears to be feasible and safe.
Placing the skin incision in the triangle of auscultation, with the anatomical localisation bounded with latissimus and trapezius muscles, and often without a muscle layer at its bottom other than intercostal muscles, makes this approach comparable to the “classical”, antero-lateral approach in regards to the muscle layers that need to be passed before reaching the pleural space.
However, puVATS approach may be even better than conventional uVATS regarding exposure of the bronchovascular structures of the ‘posterior’ segments. Moreover local and mediastinal lymphadenectomy seems to be easier with access directly in front of the entrance and at the same time in front of the lung rather than behind it.
Lobectomy can also be performed or completed from the posterior approach when necessary. Five out of six lobectomies in this cohort were performed due to oncological reasons as an extension of intended segmentectomy. In five cases, lobectomy was required as the resection margin was not clean, or without enough distance from tumor invasion. In one case there was uncertainty regarding a possible invasion of the descending aorta, for which the posterior approach was chosen. In this case however, the tumor infiltrated only the mediastinal pleura and after dissecting the tumor from aorta, it could be completely resected with a left lower lobectomy.
The primary impediment with this approach may be the width of the intercostal space. It is well known that the ribs are more narrow going antero-posteriorly, which may imply that a bigger incision is required on the posterior for removal of the specimen. To address this issue, we have found that positioning of the patient on the operation table is even more important than in the “classical” approach. The incision area should be at the highest possible level of the patient’s surface, together with a slight bending of the patient ventrally (Figure 2). This position widens the intercostal space, allowing the removal of the lung segment, and depending on the tumor size, may even be large enough to remove the entire lobe of the lung. The largest tumor which was removed in this cohort was a 4.5-cm large metastasis of colon carcinoma (10). In this case, the segment of the lung was removed without requiring any enlargement of the skin incision. The largest tumor requiring a lobectomy was removed without enlarging the incision (4 cm) was 3.7 cm large.
Median BMI of this cohort was 29. Additionally, these patients were overall rather multi morbid, having a median ACCI score of 10. Despite these issues, no significant postoperative complications were seen.
Two patients continued to smoke until the operation day. Both of these patients developed a postoperative increase in inflammatory parameters in the blood, together with an increase in bronchial secretions, yet without radiological signs of pneumonia. They were recovered well and were discharged on POD 8. One patient developed bronchitis and another one developed acute heart failure, both treated medically and were also discharged on POD 8.
There were no prolonged air leak in this cohort. Only one patient, who underwent a left upper lobectomy had an air leak which was ceased spontaneously on POD 3. All of the procedures were performed with the “fissureless” technique, without dissecting in the fissure, if the fissure was absent and the pulmonary artery could not be easily reached through it.
Median operative time seemed to be acceptable, being 160 minutes (IQR 142–178 minutes), especially considering that in four of six lobectomies a complete anatomical segment resection was performed prior to completion lobectomy. In one case, incomplete segment resection was aborted due to the intraoperative findings and the patient underwent a lobectomy.
Even postoperative pain level on the POD 1, POD 3 and at discharge seemed to be within the previous published values (11,12). Median consumption of oxycodone was 50 mg, representing nearly three days of consum at the standard dosage (10 mg BID).
In conclusion, puVATS technique appears to be a valuable method for the minimally invasive resections of the posterior segments of the lungs, together with a complete lymphadenectomy if needed. This approach showed no adverse effects, moreover, ‘fissureless’ VATS resections can also be performed easily with this technique, minimizing the potential risk of prolonged air leak.
There is no single best or the most effective approach in minimal invasive surgery (13,14), and puVATS should be taken just as a another tool aimed to facilitate utilization of uVATS at appropriate indications.
Acknowledgements
We are deeply thankful to Mrs. Peggy McLaughlin for her contribution in editing this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethical Committee on human research of the Heidelberg Medical School approved the retrospective evaluation of the data in anonymous fashion (S-430/2017). Informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, de la Torre M, et al. Bronchovascular right upper lobe reconstruction by uniportal video-assisted thoracoscopic surgery. J Thorac Dis 2014;6:861-3. [PubMed]
- Wang GS, Wang J, Rao ZP, et al. Uniportal complete video-assisted thoracoscopic surgery lobectomy with partial pulmonary arterioplasty for lung cancer with calcified lymph node. J Thorac Dis 2015;7:2366-70. [PubMed]
- Andrade H, Joubert P, Vieira A, et al. Single-port right upper lobe sleeve lobectomy for a typical carcinoid tumour. Interact Cardiovasc Thorac Surg 2017;24:315-6. [PubMed]
- Sayeed RA, Darling GE. Surface anatomy and surface landmarks for thoracic surgery. Thorac Surg Clin 2007;17:449-61. v. [Crossref] [PubMed]
- Naidu BV, Rajesh PB. Relevant surgical anatomy of the chest wall. Thorac Surg Clin 2010;20:453-63. [Crossref] [PubMed]
- Miller JI Jr. Muscles of the chest wall. Thorac Surg Clin 2007;17:463-72. [Crossref] [PubMed]
- Stamenovic D, Bostanci K, Messerschmidt A. Posterior uniportal video-assisted thoracoscopic surgery for anatomical lung resections. Asvide 2017;4:581. Available online: http://www.asvide.com/articles/1906
- Stamenovic D, Messerschmidt A. Posterior uniportal video-assisted thoracoscopic surgery for resection of the apical segment of the right lower lobe followed by completion lobectomy. Interact Cardiovasc Thorac Surg 2017;24:644-5. [PubMed]
- Stamenovic D. Uniportal Posterior Approach for Videothoracoscopic Anatomical Resection of Apical Segment of the Left Lower Lobe. CTSNet 2016; Published 16 May 2016, accessed 18.01.2017.
- Mier JM, Chavarin A, Izquierdo-Vidal C, et al. A prospective study comparing three-port video-assisted thoracoscopy with the single-incision laparoscopic surgery (SILS) port and instruments for the video thoracoscopic approach: a pilot study. Surg Endosc 2013;27:2557-60. [Crossref] [PubMed]
- Perna V, Carvajal AF, Torrecilla JA, et al. Uniportal video-assisted thoracoscopic lobectomy versus other video-assisted thoracoscopic lobectomy techniques: a randomized study. Eur J Cardiothorac Surg 2016;50:411-5. [Crossref] [PubMed]
- Migliore M. Video-assisted thoracic surgery techniques for lung cancer: which is better? Future Oncol 2016;12:1-4. [Crossref] [PubMed]
- Treasure T, Macbeth F, Russell C. If no difference in effectiveness is found between two treatments it may be because the treatments are similarly ineffective. Ann Transl Med 2015;3:201. [PubMed]


