To vent or not on veno-arterial extracorporeal membrane oxygenation, does it improve myocardial recovery and outcome?
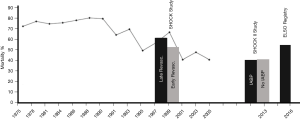
Worldwide the utilization of veno-arterial (VA) extracorporeal membrane oxygenation (ECMO) increased dramatically over the last decade, but mortality for cardiogenic shock did not significantly change and is still ranging between 50–70% (Figure 1) (5,6). Early revascularization has been shown to improve outcome in shock patients (SHOCK trial) (1). Likewise, a marked survival benefit has been documented for VA ECMO assisted resuscitation (ECPR) (7,8). However, with regard to ECMO therapy beyond CPR many questions aiming at optimization of outcome results remain unanswered so far. One of these ongoing controversies is the need of a vent during ECMO support.
Experimental evidence exists that VA ECMO induces left ventricular (LV) dysfunction with increased LV end-diastolic and systolic volumes as well as increased myocardial wall stress (9). In pediatric patients LV distension can be detrimental, but does this also apply to the adult population? Some physicians in the ECMO community are convinced of the necessity to vent the left ventricle, other colleagues are more restrictive in its use. The indication to vent the left ventricle is mainly based on clinical, echocardiographic, and radiological findings of impaired LV unloading or LV stasis and pulmonary edema. The dilemma is that at the beginning of support (especially after ECPR) more or less all patients fulfill these indication criteria. Should we vent them all to improve outcome? We basically do not know, since there is a broad lack of evidence.
Nevertheless, we know a variety of different techniques to unload the left ventricle on VA ECMO (10,11). A vent can be placed in the left atrium or ventricle with various surgical techniques. An easier and probably more frequently applied way is the intra-aortic balloon pump decompressing the left ventricle to an unknown degree by afterload reduction (12,13). Considerably more expensive is the method to drain the left ventricle with an Impella® (Abiomed, USA) pump concomitant to peripheral ECMO support. Exactly this method was investigated by Truby et al. published earlier this year in ASAIO Journal, addressing timing as well as effectiveness of LV decompression with an IMPELLA® pump (14). LV distention with the need to decompress was defined as pulmonary edema and a diastolic pulmonary artery pressure greater than 25 mmHg as a surrogate marker for LV end-diastolic-pressure. 121 patients were included in this study, of whom 7% developed LV distention requiring immediate decompression according to their definition. Another 22% of patients developed LV distension without the need to decompress the left ventricle. Survival between the groups with severe, mild and no distension on ECMO was similar. It remains unclear, whether the survival would have been worse without venting the left ventricle, since it appeared that those patients with LV distension and decompression by the IMPELLA® pump achieved lower pulmonary artery pressures compared to those patients who had no signs of LV distension. Thus, the extrapolation the lower the pulmonary artery pressure the higher the recovery or survival can’t be drawn. Interestingly, a significant improvement in pulmonary artery pressures was monitored as soon as 6 hours of support. The patients who were vented on ECMO had the lowest chance of myocardial recovery. To summarize Truby et al. study, although a smart approach was chosen by adding the parameter pulmonary artery pressure to indicate venting on ECMO a marked improvement in survival or myocardial recovery was not seen in their retrospective study. It is noteworthy that this study was the first to propose a definition of LV distension based upon clinical, hemodynamic, quantitative pulmonary artery pressures as well.
The largest study so far describing the use of the IMPELLA® pump concomitantly to VA ECMO like Truby et al. was published last year by Pappalardo et al. including 157 VA ECMO patients (15). The indication to vent the LV was seen in 34 patients (21%) without describing a clear indication to vent the LV. Notably, Pappalardo et al. describe a significant survival benefit for patients who were vented with the IMPELLA on ECMO after applying a propensity score matching analysis. The major drawback of a propensity score analysis is that many patients had been excluded by employing this statistical tool. By applying the propensity score analysis, Pappalardo’s patient cohort was broken down to 63 patients or approximately 50% of the entire initial population comparing 21 patients treated with ECMO and Impella® with 42 patients who were placed on ECMO alone.
Our ECMO center follows the strategy “Less is More” on VA ECMO. By adding another mechanical therapy to ECMO the complexity of the case increases as well as the rate of complications, at least statistically. The rate of venting in our VA ECMO population consisting of more than 600 patients is less than 2%. The concomitant use of an intraaortic balloon pump (IABP) with ECMO as the easiest method to decompress the left ventricle is not part of our protocol, neither is the use of an Impella device. The overall survival rate to discharge of our entire cohort including nearly 50% of ECPR cases is 37% in accordance with the Extracorporeal Life Support Organization (ELSO) registry and similar to the published VA ECMO literature (16). The low rate of LV venting in our population is explained by our policy of strict afterload reduction, accepting a mean arterial pressure of <50 mmHg even with no pulsatility for a period of 24 h and sometimes even longer, low flow rates of 3–4 L/min as long as lactate levels decrease, a restrictive fluid management, as well as the application of higher positive end expiratory pressure values to decrease pulmonary edema. When it comes to the question to vent or not we consider the probability of recovery. In general, it is difficult to predict the recovery potential of a patient on ECMO. However, according to our experience, there are some factors enabling judgment of recovery potential. First, the underlying heart disease implies a certain degree of recovery potential. A patient experiencing an acute myocarditis is associated with a high recovery potential, whereas patients suffering from an end-stage dilative cardiomyopathy are not (17,18). One may state that all acute but treatable diseases (e.g., myocarditis, recanalized acute myocardial infarction) have a higher recovery potential compared with end-stage diseases, such as chronic heart failure or uncorrected structural heart diseases. Second, the effectiveness of support and the end-organ response to the O2 delivery provide further hints. Patients with prolonged low venous saturations with long-lasting elevated lactate levels and persistent acidosis have a low recovery potential, whereas patients with immediate normalization of arterial blood gases have a high recovery potential. Third, echocardiography helps to stratify the recovery potential. Patients with an improving ventricular function on ECMO during the first 24 h have a much better recovery potential than patients with highly dilated ventricles and no ejection, closed aortic valve, and ventricular stasis over 24 h. In this latter patient group recovery is very unlikely even if venting is applied according to our experience. This patient population needs an urgent transition to other types of ventricular support like transplantation or implantation of a permanent assist system in case of no contraindication (e.g., neurologic impairment). In patients in whom a high recovery probability is assumed due to the above-mentioned blood gases for example, venting the left ventricle could be futile without providing further benefit.
In conclusion, it is very difficult to compare different studies and centers because of different patient populations, different managements, different indications etc. Thus, a true and probably illusionary prospective randomized study is necessary to clarify the need to vent the left ventricle on VA ECMO to answer the still unresolved title question: does venting improve recovery and outcome?
Acknowledgements
The entire ECMO Team Regensburg and all dedicated nurses and physicians at our center.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med 1999;341:625-34. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann F, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96. [Crossref] [PubMed]
- Extracorporeal Life Support Organization. International Summary. Available online: www.elso.org
- Goldberg RJ, Spencer FA, Gore JM, et al. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation 2009;119:1211-9. [Crossref] [PubMed]
- Karagiannidis C, Brodie D, Strassmann S, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med 2016;42:889-96. [Crossref] [PubMed]
- Werdan K, Gielen S, Ebelt H, et al. Mechanical circulatory support in cardiogenic shock. Eur Heart J 2014;35:156-67. [Crossref] [PubMed]
- Lin JW, Wang MJ, Yu HY, et al. Comparing the survival between extracorporeal rescue and conventional resuscitation in adult in-hospital cardiac arrests: propensity analysis of three-year data. Resuscitation 2010;81:796-803. [Crossref] [PubMed]
- Wang GN, Chen XF, Qiao L, et al. Comparison of extracorporeal and conventional cardiopulmonary resuscitation: A meta-analysis of 2 260 patients with cardiac arrest. World J Emerg Med 2017;8:5-11. [Crossref] [PubMed]
- Schiller P, Vikholm P, Hellgren L. Experimental venoarterial extracorporeal membrane oxygenation induces left ventricular dysfunction. ASAIO J 2016;62:518-24. [Crossref] [PubMed]
- Rupprecht L, Flörchinger B, Schopka S, et al. daCardiac decompression on extracorporeal life support: a review and discussion of the literature. ASAIO J 2013;59:547-53. [Crossref] [PubMed]
- Meani P, Gelsomino S, Natour E, et al. Modalities and effects of left ventricle unloading on extracorporeal life support: a review of the current literature. Eur J Heart Fail 2017;19 Suppl 2:84-91. [Crossref] [PubMed]
- Cheng R, Hachamovitch R, Makkar R, et al. Lack of survival benefit found with use of intraaortic balloon pump in extracorporeal membrane oxygenation: a pooled experience of 1,517 patients. J Invasive Cardiol 2015;27:453-8. [PubMed]
- Petroni T, Harrois A, Amour J, et al. Intra-aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Crit Care Med 2014;42:2075-82. [Crossref] [PubMed]
- Truby LK, Takeda K, Mauro C, et al. Incidence and implications of left ventricular distention during venoarterial extracorporeal membrane oxygenation support. ASAIO J 2017;63:257-65. [Crossref] [PubMed]
- Pappalardo F, Schulte C, Pieri M, et al. Concomitant implantation of Impella(®) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 2017;19:404-12. [Crossref] [PubMed]
- Camboni D, Philipp A, Rottenkolber V, et al. Long-term survival and quality of life after extracorporeal life support: a 10-year report. Eur J Cardiothorac Surg 2017;52:241-7. [Crossref] [PubMed]
- Atluri P, Ullery BW, MacArthur JW, et al. Rapid onset of fulminant myocarditis portends a favourable prognosis and the ability to bridge mechanical circulatory support to recovery. Eur J Cardiothorac Surg 2013;43:379-82. [Crossref] [PubMed]
- Ghelani SJ, Spaeder MC, Pastor W, et al. Demographics, trends, and outcomes in pediatric acute myocarditis in the United States, 2006 to 2011. Circ Cardiovasc Qual Outcomes 2012;5:622-7. [Crossref] [PubMed]


