Longitudinal study of esophageal mucosal damage after esophagectomy and gastric interposition: relationship between reflux-related mucosal injury and Notch signaling
Introduction
Gastroesophageal reflux disorder (GERD), a condition wherein the reflux of stomach contents cause reflux symptoms or/and complications, is a common disorder all over the world (1,2). GERD has clinical importance for its association with chronic esophageal injury and Barrett’s esophagus, which confers a predisposition to esophageal adenocarcinoma. In patients with chronic esophageal mucosal damage caused by gastroesophageal reflux, Barrett’s metaplasia develops when columnar cells replace esophageal squamous cells. To date, molecular pathogenesis of this process is largely unknown. Current hypotheses suggest that gastroesophageal reflux might alter the expression of some key transcription factors, leading to transdifferentiation from mature esophageal squamous cells to mature columnar cells or inducing immature esophageal progenitor cells to undergo columnar differentiation (3-5).
Notch signaling, a group of highly conserved type I transmembrane glycoproteins, is widely involved in cell development, differentiation, proliferation and apoptosis. It is generally considered as one of the most important signaling pathways for cell fate determination (6-8). Some studies have shown that Notch signaling plays a critical role in cell differentiation towards a secretory cell lineage, such as goblet cells. Active Notch signaling could preserve basal cells of epithelium from differentiation, while inhibition of Notch signals promoted secretory cells production (9,10). In vitro experiments provided evidence that inhibition of Notch signaling induced by bile acid was correlated with the formation of Barrett’s esophagus (11,12). Via 3D organotypic culture technique, a study reported inhibition of Notch signaling could enhance transdifferentiation of esophageal squamous epithelium towards a Barrett’s metaplasia (13). Nevertheless, Notch signaling pathway has not been investigated in human model of gastroesophageal reflux. For a more accurate understanding of molecular pathogenesis of Barrett esophagus, it is necessary to study Notch signaling in an ideal human gastroesophageal reflux model.
Esophagectomy and esophagogastrostomy is usually indicated for patients with resectable esophageal cancer. Numerous studies have proven that reflux is a common complication after esophagectomy, and esophageal mucosal injury cause by reflux would eventually develop in majority of patients (14,15). Accordingly, esophagectomy and esophagogastrostomy creates a good gastroesophageal reflux model to study molecular pathogenesis of reflux-induced esophageal mucosal damage. In the present study, we utilized this model to investigate the role of Notch signaling in the development of esophageal mucosal damage induced by reflux.
Methods
Study population and follow-up
From February 2011 to December 2012, patients undergoing esophagectomy with gastric interposition were selected for this prospective study. This work was approved by the ethics committee of West China Hospital (WCH2010-56) and informed consent was obtained from all patients. All experiments were performed in accordance with the relevant guidelines and regulations.
To allow a longer follow-up period in this longitudinal study, we included patients with early stage esophageal squamous cell carcinoma (Union for International Cancer Control, Esophageal Cancer TNM staging 7th Edition, Tis or T1-2N0M0). Standard Ivor-Lewis esophagectomy without pyloroplasty or pylorotomy was performed, and intrathoracic anastomoses were made by hand-sewn technique with an end-to-side configuration. We only included patients free of the postoperative anastomotic complications (i.e., leakage and stricture formation). Patients with residual tumor at the esophageal stump after surgery or with tumor recurrence during the follow-up period were excluded from this study.
During operation, two pieces of normal esophageal mucosa from resected specimen were obtained, one was flash frozen for polymerase chain reaction (PCR), and the other was fixed into formalin for immunohistochemistry (IHC). Patients after esophagectomy were routinely followed up every 3 months for the first year, every 6 months for the second year and then annually. For this study, postoperative follow-up examinations were scheduled at 6, 18, 36 and 48 months. Reflux symptom assessment, endoscopic evaluation of esophageal mucosal damage and biopsy collection were performed. Proton pump inhibitor (PPI) and prokinetics were indicated for patients with significant reflux symptoms or complications.
Reflux symptoms
Postoperative symptoms for gastroesophageal reflux included: dysphagia, regurgitation, heartburn and odynophagia. Some other gastroesophageal reflux related symptoms were also taken into consideration, such as cough, asthma, aspiration and laryngitis. The intensity of reflux was scored as follows: 0, no reflux on semi-supine position; 1, postprandial reflux on semi-supine position; 2, reflux with empty stomach on semi-supine position; 3, postprandial reflux on upright position; 4, reflux with empty stomach on upright position (16). Besides, medications (PPTs and/or prokinetics) for the treatment of reflux were recorded.
Endoscopic assessment and histology
Endoscopic examinations of the esophageal remnant were performed in all patients using an Olympus system. Description of the mucosal damage caused by reflux was based on Armstrong et al.’s MUSE system. The lesions were classified into metaplasia (M), ulcer (U), stricture (S), and erosion (E). Besides, the severity of each lesion was scored as follows: 0, no; 1, mild; 2, severe. Score 0 was considered MUSE negative, and score 1 or greater was considered MUSE positive (17). The location of the anastomotic site was recorded as the distance from the incisors, and the gastric conduit was then examined with recording of the evidence of duodenogastric reflux. Endoscopic bile reflux was recorded when bile colored liquid present in gastric cavity. Esophagogastric anastomosis is easily visible as a marker of the new gastro-esophageal junction when performing endoscopy. Four circumferential biopsies were taken by conventional forceps from suspected area of the esophageal remnant or at a distance of 2 cm away from the anastomotic site when there was no damage visualized. Two biopsies were fixed in formalin immediately and then embedded in paraffin for IHC or hematoxylin and eosin staining, and the others were flash frozen by liquid nitrogen and then stored at −80 °C for later RNA extraction and PCR. Histological evaluation of esophageal remnant mucosa was done on sections with hematoxylin and eosin staining by independent pathologists. The esophageal remnant damage caused by reflux was reviewed as for idiopathic reflux disease, including inflammation, mucosal breaks (erosion or ulceration), and columnar lined metaplasia (15). The histological interpretations were correlated with endoscopic visual assessment of mucosal damage.
IHC
IHC staining was performed to detect proteins expression using formalin-fixed, paraffin-embedded block. Sections were deparaffinized with xylene and rehydrated through sequential graded ethanol washes. Endogenous peroxidase was blocked by incubation with 3% hydrogen peroxide. After antigen retrieval, slides were incubated with primary antibodies (Notch1, Santa Cruz, 1:100 dilution; Hes1, abcam, 1:200 dilution), followed by incubation with biotinylated secondary antibody (Dako, 1:300 dilution). The antibodies were previously validated by western blot. The bound antibodies were detected through an avidin-biotin-peroxidase method and DAB. Finally, the slides were counterstained with Harris Hematoxylin and mounted with coverslips. Known positive controls (normal skin tissue) were included in each run and negative controls were done by omitting the primary antibodies. The results were interpreted using semi-quantification via the three-grade system, where 0 denoted absence of staining; 1 denoted minimal and variable staining; 2 denoted obvious and intense staining. Only sections with grade 2 were considered positive staining. Two independent observers accessed the sections for the immunohistochemical staining; any disagreement on scoring was resolved by discussion or arbitrated by another. The observers were blinded regarding the clinical characteristics of the patients.
Real-time PCR
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The quantification and qualification of total RNA were determined with Agilent’s 2100 Bioanalyzer (Agilent, Wilmington, DE, USA) using RNA 6000 Nano Assay Kit (Agilent, Wilmington, DE, USA). Complementary DNA (cDNA) was prepared using the QuantiTect Reverse Transcription kit (Qiagen, Hilden, Germany), according to the instructions. The concentration and quality of the cDNA samples were measured by NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). For real-time quantitative PCR, a serial dilution for each gene was performed to construct the standard curve. Five µL of cDNA (synthesized from 1 µg total RNA) and 1 µL of primers (10 pmol/µL) were mixed with SYBR Green Master Mix using QuantiTect SYBR Green PCR kit in final reaction volume of 25 µL. The RT-PCR was performed on Rotor Gene 3000, each assay was performed in triplicate and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as reference gene. The sequences and amplicon size for the primers are listed in Table 1. PCR products were validated by melting curve analysis and agarose gel electrophoresis. The relative quantification of expression was calculated with double-standard curve method, and the ratio of a target gene is expressed in a sample versus a control in comparison to a reference gene (GAPDH) (18).

Full table
Statistical analysis
Qualitative data were reported as frequencies and percentages, continuous data were described as median with interquartile range. One-way ANOVA and paired t-test were used to compare differences in mRNA levels at different time points. The mRNA levels in different histological groups were compared by using the Kruskal–Wallis test. Friedman’s test was used to compare ordinal data (reflux score). The Chi square test was used to analyze categorical data. Correlation analysis was performed between Notch1 and Hes1 expression levels with Pearson’s correlation coefficient. The difference was considered statistically significant when P value <0.05. For consideration of the effect of multiple testing, Bonferroni correction was used to adjust the cutoff P value for the significance of multiple comparisons. P values below 0.005 were considered to indicate statistical significance when five different time points were compared. Statistical analyses were carried out using SPSS Statistics version 19.0 (SPSS IBM Inc., Chicago, IL, USA).
Results
Patients
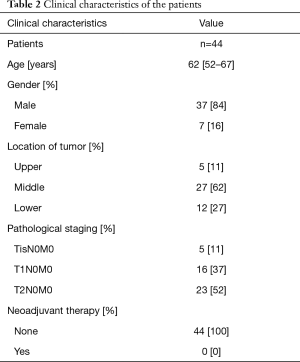
Forty-eight patients who underwent Ivor-Lewis esophagectomy for esophageal squamous cell carcinoma were initially included in this prospective longitudinal study. During the study period, 3 patients were excluded from the study for the tumor recurrence and 1 patient was lost to follow-up, giving that 44 out of 48 patients (92%) completed 4-year follow-up. The patient characteristics and clinical data are summarized in Table 2. Preoperative diagnosis and clinical staging were reached by upper gastrointestinal endoscopy with biopsies and contrast-enhanced computerized tomography. All patients had no history of gastroesophageal reflux disease or endoscopic evidence of reflux-related esophageal mucosal damage before operation. They received no perioperative adjuvant therapy for esophageal cancer, and the postoperative pathological staging was performed in accordance with the guidelines by 7th Edition of Esophageal Cancer TNM staging. There were no peri-operative deaths and patients were followed up for more than 4 years. Symptoms of reflux and endoscopic visualized reflux-related mucosal damage of the remnant esophagus were evaluated at every follow-up. Meanwhile, biopsies were obtained for histological examination, IHC and PCR analyses.
Full table
Evaluation of reflux symptoms
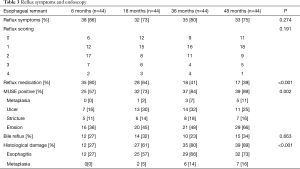
Reflux was a common complication for patients after esophagectomy with gastric interposition, and regurgitation presented as the most common symptom for postoperative reflux (Table 3). Gastroesophageal reflux associated symptoms were seen in 86% of patients at 6 months, 73% of patients at 18 months, 80% of patients at 36 months and 75% of patients at 48 months after operation, and there was no significant difference between the time points (P=0.274). With respect to the reflux intensity scoring, no statistical difference could be detected for the four follow-ups (P=0.191). However, the number of patients who received reflux medication decreased with time continuously following surgery, and the difference reached statistical significance (P<0.001).
Full table
Endoscopy
Endoscopic assessment and biopsies were performed at each follow-up, and 176 endoscopic examinations were undertaken. Description of the mucosal damage caused by reflux was based on Armstrong et al.’s MUSE system (17). Endoscopic findings are described in Table 3. Images were taken for the confirmation of the esophageal remnant mucosal damage (Figure 1). The anastomosis was measured at 22.5±3.6 cm from the incisors in this cohort. The reflux associated injuries of esophageal remnant were endoscopically visualized (MUSE positive) more often in later follow-ups, and the MUSE positive rate increased from 57% at 6 months to 89% at 48 months (P=0.002). Erosion represented the most common visualized damage in esophageal remnant, which was documented in 75 of the 176 examinations (43%). It is notable that bile reflux was a common event for patients after esophagectomy and esophagogastrostomy as well (27%, 32%, 23%, and 34% for 6, 18, 36 and 48 months, respectively), while there were no significant differences among different follow-ups (P=0.663).
Histological assessment
Histological assessment of esophageal remnant revealed that reflux associated damage increased significantly from 27% of patients at 6 months to 89% at 48 months after operation (P<0.001), which was in accordance with the trend of endoscopically visualized damage. Among the 176 biopsies taken for histological assessment, 63 (36%) samples demonstrated no abnormalities. For the remaining 113 samples, 98 (56%) presented esophagitis (inflammation or mucosal breaks) and 15 (9%) developed metaplasia. There was an increasing prevalence of columnar metaplasia along the study period. The initial detection of columnar metaplasia was made at 18 months (5%), and the percentage of metaplasia increased to 14% at 36 months and 16% at 48 months. None of the columnar metaplasia progressed to dysplasia during the study period. There were no correlations detected between reflux symptoms and documented mucosal damage including endoscopic or histological evidence (P>0.05).
Expressions of Notch1 and Hes1 mRNA
The relative expression levels of Notch1 and Hes1 mRNA in esophageal specimens from different time points are shown in Figure 2. All biopsies including normal esophagus, esophagitis and metaplasia were included for each follow-up time point. The results indicated that expression of Notch1 decreased in a time-dependent manner after operation, and the difference reached statistical significance (P<0.001). We also applied the pairwise comparisons to the five different groups, exhibiting a decreasing pattern from “operation” to “6 months”, from “6 months” to “18 months”, from “18 months” to “36 months” (P<0.001), but not from “36 months” to “48 months” (P=0.068). When comparing the expression levels of Notch1 mRNA in samples with and without endoscopic evidence of mucosal damage, MUSE positive samples demonstrated lower expression as compared with MUSE negative biopsies (P=0.035). As for the Notch1 mRNA expression in esophageal mucosa with different histological alterations, significant differences were detected among normal squamous mucosa, esophagitis and metaplasia (P<0.001) (Figure 3A). Normal squamous epithelium specimens showed significantly higher expression of Notch1 mRNA as compared to esophagitis (P=0.036), and Notch1 mRNA expression was also higher in esophagitis than in metaplasia (P=0.004).
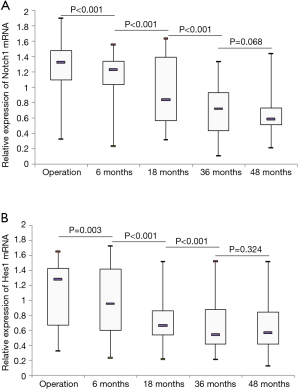
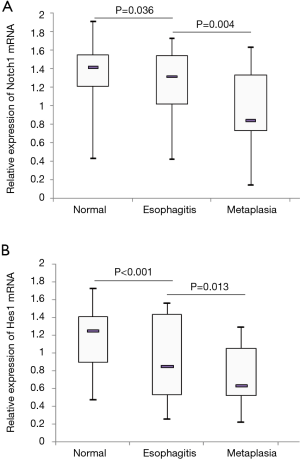
Hes1 is a well characterized downstream target gene for Notch signaling. Thus, we also investigated the expression of Hes1 mRNA in the biopsy samples. It turned out that Hes1 mRNA level decreased in a time-dependent manner. The expression levels of Notch1 and Hes1 at five time points were well correlated with each other (P<0.001). The Pearson correlation coefficients for the five time points were 0.582, 0.740, 0.924, 0.797 and 0.912, respectively. The comparisons between different groups also showed a decreasing pattern from “operation” to “6 months” (P=0.003), from “6 months” to “18 months” (P<0.001), from “18 months” to “36 months” (P<0.001), but not from “36 months” to “48 months” (P=0.324). Samples with endoscopic evidence of mucosal damage (MUSE positive) demonstrated lower level of Hes1 mRNA expression as compared to biopsies with normal endoscopic findings (MUSE negative) (P=0.017). Hes1 expression levels for normal squamous epithelium, esophagitis and columnar metaplasia are displayed in Figure 3B. The difference among the three histological groups was statistically significant (P<0.001). Histologically normal squamous epithelium exhibited higher expression of Hes1 mRNA when compared with histologically confirmed esophagitis (P<0.001), and significant lower expression of Hes1 mRNA was detected in metaplasia compared to esophagitis (P=0.013). However, no significant differences were observed in Notch1 or Hes1 mRNA expression between samples with and without endoscopically evident bile reflux (P>0.05). To assess the possibility that the changes in Notch1/Hes1 were consequence of degree of reflux injury and not time-dependent, we compared Notch1/Hes1 expression for each histological alteration including normal esophagus, esophagitis and metaplasia among different time points. It turned out that there were no significant differences among different time points (P>0.05).
Immunohistochemical examination of Notch1 and Hes1
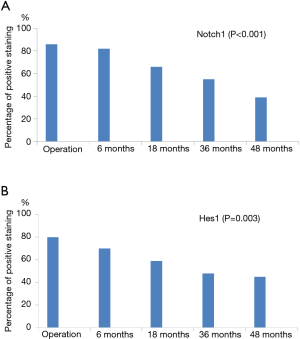
IHC was also performed to investigate expressions of Notch1 and Hes1 in surgical and postoperative endoscopic biopsy specimens. Notch1 expression was typically localized in cell membrane and cytoplasm of esophageal epithelial cells (Figure 4), and Hes1 expression was found in cytoplasm and nucleus (Figure 5). Notch1 and Hes1 positive stainings were more commonly seen in sections from surgical specimens as compared to specimens from postoperative biopsies. When we compared sections at different time points after esophagectomy, there was a decreasing trend of the expression of Notch1 (P<0.001) and Hes1 (P=0.003) staining in esophageal mucosa exposure to long-term postoperative reflux (Figure 6). As for the samples with different histological alterations, both Notch1 and Hes1 presented a decreasing expression pattern from normal mucosa to esophagitis and metaplasia, the differences among groups reached statistical significance (P<0.001). Nevertheless, no significant differences in Notch1 or Hes1 protein expression were revealed for the following comparisons: MUSE positive and MUSE negative specimens, samples with and without reflux symptom, biopsies with and without evidence of bile reflux (P>0.05).


Discussion
Gastric interposition has remained the preferred way for digestive reconstruction after esophagectomy. Independently of the approaches used for esophageal reconstruction, esophagectomy with gastric interposition destroys most defense mechanisms against reflux, creating a communicating cavity between the stomach and the esophagus. Gastroesophageal reflux is a common complication after esophagectomy and esophagogastrostomy, which could eventually result in damage to esophageal remnant. Several studies have reported that esophagectomy with gastric pull-up would increase the development of esophagitis and also Barrett’s epithelium (19-21). Therefore, esophagectomy with gastric interposition may represent a good in vivo model for the pathogenesis of GERD. To the best of our knowledge, this is the first report that prospectively investigated Notch signaling in human model of gastroesophageal reflux over a long period of time.
In our study, esophagectomy with intrathoracic esophagogastrostomy was indicated for patients with early stage squamous cell carcinoma, without any chemotherapy or radiotherapy, which is important for the investigation of molecular pathogenesis of reflux-related mucosal damage in vivo model. During the long term follow-up period of 4 years, the majority of patients complained of significant reflux symptoms, and endoscopic or histological evidence of mucosal damage was detected in most of those patients. We have shown mucosal damage was more often observed with longer follow-up when assessed endoscopically or histologically. However, we found no correlation between reflux symptoms and endoscopically visualized damage or histological results. Symptom-based assessment of gastroesophageal reflux has major limitations; endoscopy and biopsies are more reliable methods to evaluate the esophageal remnant after esophagogastrostomy. Some reports even consider that reflux symptoms actually have no value in predicting the existence of reflux-related injury in esophageal remnant (15,22). Notably, 16% of patients in our longitudinal study developed metaplasia in the remnant esophagus, the prevalence was much less than that in western countries (20,23). We previously investigated the difference of mucosal damage in the esophageal remnant after esophagectomy and gastric interposition between Chinese and Canadian population, revealing that Canadian population was more sensitive to the gastroesophageal reflux damage compared to Chinese population, which might be attributed to the different genetic backgrounds (24).
In vitro studies suggest that Notch signaling inhibition could promote transdifferentiation of the esophageal squamous epithelium toward Barrett’s-like metaplasia (11,13). Moreover, in a rat model, inhibition of Notch signaling by gamma-secretase inhibitors resulted in intestinal goblet cell metaplasia (25). In our study, Notch1 mRNA expression in reflux-exposed squamous epithelium decreased in a time-dependent manner after esophagectomy and esophagogastrostomy, and that of Hes1, as the downstream target gene of Notch signaling, exhibited the same pattern. Immunohistochemical studies also confirmed Notch1 and Hes1 expressions were suppressed in a similar pattern after esophagectomy. Given that gastroesophageal reflux has been well documented to develop in almost all patients after esophagectomy with gastric interposition, our longitudinal study from human model indicated that reflux to the esophageal remnant might inhibit Notch signaling pathway.
This in vivo study also provided evidence supporting that Notch signaling pathway might have an important role in the process of mucosal damage after gastroesophageal reflux. A large portion of esophageal mucosa near the anastomotic region showed some degree of endoscopically visualized or histologically confirmed damage after operation. From normal squamous epithelium to reflux esophagitis and metaplasia, expression levels of Notch1 were progressively decreased when histological alterations caused by reflux progressed, particularly when metaplasia was present. As the Notch signaling downstream target gene, Hes1 followed the same trend, supporting that Notch signaling is involved in the pathogenesis of reflux-related mucosa damage.
In addition, Notch expression levels in samples with and without endoscopically visualized damage were compared. MUSE positive samples demonstrated lower expression of Notch mRNA as compared with MUSE negative biopsies. We anticipated observing less expression of Notch protein as assessed by IHC for MUSE positive specimens. However, no significant difference was found between groups. We also noted that there was no significant correlation between endoscopic findings and histological evidence of mucosal damage, demonstrating endoscopic findings could not truly reflect the actual severity of the mucosal damage caused by reflux. In our study, four biopsies were collected using conventional forceps; two for HE and IHC, and two for PCR. Besides sampling error, the presence of nonerosive reflux disease could be the other possible explanation for the discrepancy between histology and endoscopy. Our observations are comparable with results from other reports (15). This might also explain why the difference of Notch protein expression between MUSE positive and MUSE negative groups did not reach statistical significance. Furthermore, we also found that prevalence of reflux damage (MUSE or histology) was generally greater at all-time points than the prevalence of Notch1/Hes1 IHC loss, there was a possibility that loss of Notch signaling was not a driver of metaplasia but was simply a consequence. More studies are demanded to elucidate this issue.
Bile acids are known to have an important role in the development of Barrett esophagus (26), and we examined their effects on the Notch expression. A report by Tamagama et al. showed that stimulation with bile acid in esophageal cell lines suppressed Notch signaling pathway (11). One of our initial goals was to verify the notion that the patients without bile reflux had a higher expression of Notch signaling. Nevertheless, bile reflux was found in 51 out of 176 endoscopic examinations, and we failed to find the effect of bile reflux on the development of mucosal damage or the Notch signaling expression. In our center, intragastric bile acids examination, hepatobiliary scintigraphy or pH manometry was not routinely performed for patients after esophagectomy, and the reflux was assessed solely by endoscopy. However, it is known that endoscopy has limited sensitivity and specificity of diagnosis of bile reflux (27), which might explain why we did not see the relationship between the bile reflux and mucosal damage or Notch expression. Another limitation about this study was that we could not confirm the histological subtype for the flash frozen biopsy used for gene expression because of the very small size of each biopsy sample. To minimize this sampling error, circumferential biopsies were taken from suspected area of the esophageal remnant or at a distance of 2 cm away from the anastomotic site when there was no damage visualized. Base on the samples used for HE or IHC staining, it seemed that they had good consistency with histological subtype. Furthermore, some patients in this study received medication therapy for controlling reflux symptoms. PPI and prokinetics were indicated for patients with significant reflux symptoms or reflux complications. However, we found that medication use decreased despite ongoing symptoms and worsening endoscopic evidence of damage. It seemed that reflux symptoms were more tolerable in patients with longer postoperative period. Seventeen patients received anti-reflux medication at 48 months, and 16 (94%) of them had histological evidence of injury; while 23 (85%) out of 27 patients without anti-reflux medication had histologically confirmed damage. The difference did not reach statistical significance (P=0.63). The medication therapy in some patients was neither on a regular basis nor long-lasting due to a low level of compliance, making it impractical to correlate it with Notch signaling expression assessment. It is still unclear whether or not medication usage could alter the expression of Notch signaling in esophageal mucosa after gastric interposition. Further molecular biology studies should clarify this issue.
Conclusions
In conclusion, reflux-related esophageal mucosal damage is a common complication in patient with esophagectomy and gastric interposition. By using this human reflux model, our longitudinal follow-up study suggested that gastroesophageal reflux might suppress the Notch signaling pathway in esophageal mucosa, and Notch signaling might play a potential role in the development of mucosa damage after gastroesophageal reflux. More functional studies are demanded to verify our preliminary findings. Future studies using this in vivo model of gastroesophageal reflux will contribute to investigating the role of key signaling pathways in the pathogenesis of Barrett esophagus.
Acknowledgements
The authors greatly appreciate Dr. Andre Duranceau (Centre Hospitalier de l’Université de Montréal, Quebec, Canada) for his expert advice on the study.
Funding: This work was supported by National Nature Science Foundation of China (grant number 81500419, 30770982).
Footnote
Conflicts of Interest: This study was accepted for oral presentation at 24th ESTS annual meeting (Naples, Italy, 2016), and was granted the ESTS-DGT Travel grant. It was also orally presented at 15th World Congress of the International Society for Diseases of the Esophagus (ISDE) (Singapore, September, 2016).
Ethical Statement: This study was approved by the ethics committee of West China Hospital (WCH2010-56) and informed consent was obtained from all patients. All experiments were performed in accordance with the relevant guidelines and regulations.
References
- Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900-20. [Crossref] [PubMed]
- Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med 2014;371:836-45. [Crossref] [PubMed]
- Croagh D, Thomas RJ, Phillips WA, et al. Esophageal stem cells--a review of their identification and characterization. Stem Cell Rev 2008;4:261-8. [Crossref] [PubMed]
- Quante M, Abrams JA, Lee Y, et al. Barrett esophagus: what a mouse model can teach us about human disease. Cell Cycle 2012;11:4328-38. [Crossref] [PubMed]
- Stamp LA, Braxton DR, Wu J, et al. The GCTM-5 epitope associated with the mucin-like glycoprotein FCGBP marks progenitor cells in tissues of endodermal origin. Stem Cells 2012;30:1999-2009. [Crossref] [PubMed]
- Fre S, Huyghe M, Mourikis P, et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature 2005;435:964-8. [Crossref] [PubMed]
- Ramasamy SK, Kusumbe AP, Wang L, et al. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014;507:376-80. [Crossref] [PubMed]
- Lobry C, Oh P, Mansour MR, et al. Notch signaling: switching an oncogene to a tumor suppressor. Blood 2014;123:2451-9. [Crossref] [PubMed]
- Katoh M. Notch signaling in gastrointestinal tract Int J Oncol 2007;30:247-51. (review). [PubMed]
- Wong GT, Manfra D, Poulet FM, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem 2004;279:12876-82. [Crossref] [PubMed]
- Tamagawa Y, Ishimura N, Uno G, et al. Notch signaling pathway and CDX2 expression in the development of Barrett's esophagus. Lab Invest 2012;92:896-909. [Crossref] [PubMed]
- Morrow DJ, Avissar NE, Toia L, et al. Pathogenesis of Barrett's esophagus: bile acids inhibit the Notch signaling pathway with induction of CDX2 gene expression in human esophageal cells. Surgery 2009;146:714-21; discussion 721-2. [Crossref] [PubMed]
- Vega ME, Giroux V, Natsuizaka M, et al. Inhibition of Notch signaling enhances transdifferentiation of the esophageal squamous epithelium towards a Barrett's-like metaplasia via KLF4. Cell Cycle 2014;13:3857-66. [Crossref] [PubMed]
- Lord RV, Wickramasinghe K, Johansson JJ, et al. Cardiac mucosa in the esophageal remnant after esophagectomy is an acquired epithelium with Barrett’s-like features. Surgery 2004;136:633-40. [Crossref] [PubMed]
- D'Journo XB, Martin J, Rakovich G, et al. Mucosal damage in the esophageal remnant after esophagectomy and gastric transposition. Ann Surg 2009;249:262-8. [Crossref] [PubMed]
- Wang WP, Gao Q, Wang KN, et al. A prospective randomized controlled trial of semi-mechanical versus hand-sewn or circular stapled esophagogastrostomy for prevention of anastomotic stricture. World J Surg 2013;37:1043-50. [Crossref] [PubMed]
- Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 1996;111:85-92. [Crossref] [PubMed]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol 2002;29:23-39. [Crossref] [PubMed]
- Dresner SM, Griffin SM, Wayman J, et al. Human model of duodenogastro-oesophageal reflux in the development of Barrett's metaplasia. Br J Surg 2003;90:1120-8. [Crossref] [PubMed]
- O'Riordan JM, Tucker ON, Byrne PJ, et al. Factors influencing the development of Barrett's epithelium in the esophageal remnant postesophagectomy. Am J Gastroenterol 2004;99:205-11. [Crossref] [PubMed]
- da Rocha JR, Ribeiro U Jr, Sallum RA, et al. Barrett's esophagus (BE) and carcinoma in the esophageal stump (ES) after esophagectomy with gastric pull-up in achalasia patients: a study based on 10 years follow-up. Ann Surg Oncol 2008;15:2903-9. [Crossref] [PubMed]
- Jones EL, Meara MP, Schwartz JS, et al. Gastroesophageal reflux symptoms do not correlate with objective pH testing after peroral endoscopic myotomy. Surg Endosc 2016;30:947-52. [Crossref] [PubMed]
- D'Journo XB, Martin J, Ferraro P, et al. The esophageal remnant after gastric interposition. Dis Esophagus 2008;21:377-88. [Crossref] [PubMed]
- Yuan Y, Duranceau A, Chen L, et al. Comparison of mucosal reflux damage in remnant esophagus after esophagectomy and gastric interposition between Chinese and Canadian population. Zhonghua Wei Chang Wai Ke Za Zhi 2015;18:871-4. [PubMed]
- Milano J, McKay J, Dagenais C, et al. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia andinduction of genes known to specify gut secretory lineage differentiation. Toxicol Sci 2004;82:341-58. [Crossref] [PubMed]
- Tamagawa Y, Ishimura N, Uno G, et al. Bile acids induce Delta-like 1 expression via Cdx2-dependent pathway in the development of Barrett's esophagus. Lab Invest 2016;96:325-37. [Crossref] [PubMed]
- Chen TF, Yadav PK, Wu RJ, et al. Comparative evaluation of intragastric bile acids and hepatobiliary scintigraphy in the diagnosis of duodenogastric reflux. World J Gastroenterol 2013;19:2187-96. [Crossref] [PubMed]


