Argatroban administration as therapy for thrombosis in patients with continuous-flow ventricular assist devices
Introduction
Left ventricular assist devices (LVAD) have improved the survival rates of patients with terminal heart failure and can be implanted to bridge patients to heart transplantation or to improve the symptoms of patients ineligible for transplantation (1-5). Currently implantation, exchange and explantation of VAD-systems can be minimally invasive, in order to reduce trauma and infection (6-10). Complications of device therapy are common and can include thrombotic and bleeding complications (11-13). Recent registry data show that up to 70% of patients will have a major complication during the first year of device support (14). Risk factors for developing device thrombosis have been identified as pump-specific such as shear stress, flow dynamics and blood trauma, as well as patient-dependent [e.g., atrial fibrillation, pre-existing atrial or ventricular thrombus, non-compliance, hypercoagulable disorders (15)], implantation technique and anticoagulation strategies (16). Typical clinical symptoms include increased pump power, anemia, recurrent heart failure and hemolysis (17). LVAD thrombosis may be confirmed by echocardiography (18), computed tomography (CT) (19) or cardiac catheterization (20). The recommended treatment of LVAD thrombosis is pump replacement or emergency heart transplantation (16,21).
Conservative thrombosis management involves intravenous (IV) therapy with heparin (15). However, the reported mortality rate of patients treated medically is 50% (22). Hence there is critical need for new strategies for LVAD patient anticoagulation management. Previously published case series have suggested the application of glycoprotein (GP)-inhibitors for suspected device obstruction (23-26), which was associated with excessive bleeding events. Investigators concluded that the risk of medical therapy with eptifibatide outweighed the benefits of GP-inhibitor application (26). Data on the application of direct thrombin inhibitors such as argatroban and bivalirudin is limited. Argatroban is a direct reversible thrombin inhibitor that interacts with free and clot-bound thrombin and inhibits the activation of coagulation factors V, VIII, XIII and protein C (27). Preliminary data from previously published case series on treating patients with device-related thrombosis with argatroban is promising (28).
The purpose of the current study was to investigate the safety and effectiveness of argatroban therapy for device-related thrombosis as an alternative to pump exchange.
Methods
Clinical data acquisition
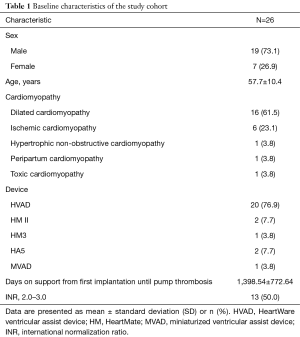
Data was collected by electronic medical record review. In this retrospective study 26 patients on VAD-therapy have been included who were admitted to our outpatient clinic between April, 2012 and February, 2017 with suspected VAD-thrombosis. Nineteen patients were on HeartWare ventricular assist device (HVAD) support, three on HeartMate II (HM II), two on HeartAssist 5 (HA5) and one on HeartMate 3 (HM3) and miniaturized ventricular assist device (MVAD). These patients were subsequently hospitalized and treated with argatroban. Criteria for suspected VAD-thrombosis were defined as either hemolysis or altered VAD-parameters, such as change in flow or motor power. Data collected until February 28th 2017 was analyzed for this study. All adverse events were determined through retrospective examination of medical records.
Argatroban administration
The argatroban dosage was started as a permanent IV infusion (2 µg/kg/min) at admission.
Statistical analysis
SPSS 23.0 package (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis of clinical data. Categorical and continuous variables were summarized as frequencies, percentages and mean/median with interquartile range, respectively. The student’s t-test was applied to compare baseline characteristics of inpatient admission and discharge with statistical significance considered for P<0.05.
Results
Patient profile prior to argatroban administration
Data from 26 patients with suspected pump thrombosis, who were treated with argatroban between April, 2012 and February, 2017 was analyzed in this study. Detailed baseline characteristics of the study group can be found in Table 1. In 73.1% of the study population was male, with a mean age of 57.7 years. In 61.5% of the study group dilated cardiomyopathy was the primary heart disease prior to LVAD implantation. In 23.1% ischemic cardiomyopathy and in 3.8% hypertrophic, non-obstructive, peripartal or toxic cardiomyopathy was the underlying disease. Mean duration of support when treated with argatroban for pump thrombosis was 1,398 days. Fifty percent of the patients in the current study exhibited deranged international normalization ratio (INR) at the time of hospital admission.
Full table
Laboratory values at the time of admission and discharge are listed in Table 2. Serum levels of free hemoglobin (fHb), glutamic-oxaloacetic transaminase (GOT), lactate and lactate dehydrogenase (LDH) at the time of admission were elevated. At the time of discharge fHb, GOT, lactate and LDH levels were significantly decreased. Furthermore, thrombocyte and creatinine level in patients were significantly higher at discharge. In 65% of the patients displayed hematuria upon hospitalization.

Full table
VAD-parameters such as flow and motor power appeared to significantly rise at the time of admission (Table 3).

Full table
Clinical outcome after argatroban therapy due to pump thrombosis
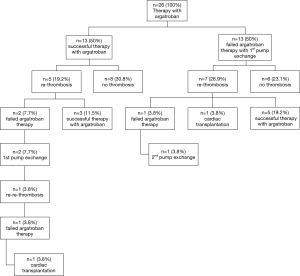
Thirteen patients (50%) displayed normalization of clinical symptoms, laboratory values and VAD-parameters, upon argatroban therapy. These 13 patients did not require further intervention and were discharged. In 5 of 13 patients recurrent thrombosis occurred after a mean duration of 217 days (minimum 30 days, maximum 648 days). Three patients displaying such recurrent thrombosis were successfully treated with argatroban therapy. In two patients VAD exchange was necessary. One patient developed re-thrombosis 7 days after the VAD exchange and received cardiac transplant (Figure 1). Eight of 13 patients (30.8%) remained free from pump thrombosis.
In the other 13 patients of the study cohort argatroban therapy failed and an exchange of the VAD-system was performed. In 26.9% of those patients developed re-thrombosis on an average of 255 days (range 14–760 days) later. In 5 patients re-thrombosis could be treated successfully with argatroban, in one patient the VAD system had to be changed again and one patient successfully received cardiac transplant. 90 days after discharge 6 of 13 patients with first VAD-exchange (23.1%) remained free of pump thrombosis (Figure 2).
Adverse events
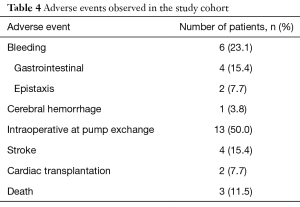
All observed adverse events are listed in Table 4. Four of all patients (15.4%) had a stroke event during the follow up period. In 11.5% the stroke occurred during the postoperative course after VAD-exchange. Hemorrhagic strokes were not observed.
Full table
Six patients of the study cohort (23.1%) had bleeding events after argatroban therapy. One patient displayed gastrointestinal bleeding six months after argatroban therapy, and needed blood transfusion. Another patient had to be re-hospitalized due to gastrointestinal bleeding only four days after discharge. This patient developed epistaxis and two major gastrointestinal bleedings during the next three months. One patient experienced five episodes of gastrointestinal bleeding, four months after therapy with argatroban. Another patient required electrocoagulation for epistaxis ten days after argatroban therapy and VAD-exchange. One patient suffered an ischemic stroke, followed by a hemorrhage 19 months after argatroban therapy.
In all patients requiring VAD-exchange due to insufficient argatroban therapy, intraoperative substitution of erythrocytes (mean of four erythrocyte concentrates) was necessary.
In total two patients have been listed for high urgency heart transplantation. One patient was transplanted due to a recurrent thrombosis after first VAD-exchange. In this patient argatroban showed both times no effect. The other heart transplanted patient was initially treated successfully with argatroban. However, the argatroban treatment of the re-thrombosis failed this time and the patient received a VAD-exchange. After the third re-thrombosis the patient has been successfully transplanted.
During the observation period 11.5% patients died. In the study population the main causes of death were multi-organ failure (3.8%) and ischemic stroke (3.8%). The cause of death of another patient is unknown.
Discussion
Pump thrombosis is a severe, life threatening complication of VAD-therapy. The gold standard for VAD thrombosis therapy is heart transplantation. Due to low availability of donor organs, the common therapy is VAD exchange (29,30). Due to perioperative complications, conservative treatment of VAD-thrombosis is an alternative option. Despite this limited data is available for alternative therapeutic strategies.
This study is the first to evaluate argatroban administration for therapy of suspected VAD-thrombosis. In our study cohort a change of VAD-parameters, laboratory values or clinical symptoms were inclusion criteria. Laboratory analyses revealed elevated levels of LDH, fHb, GOT and lactate at the time of hospital admission. Sixty-five percent of the patients also had hemoglobinuria. At the time of hospital discharge a significant decrease of all parameters was observed. Elevation at the time of admission is due to shear forces at the thrombus with destruction of erythrocytes. Decrease at discharge can be seen as result of a successful resolution of the thrombus. Further on thrombocytes and serum-creatinin-level showed to be elevated at the time of discharge. Argatroban is hepatically metabolized and has no effect on kidney function. Most patients receive a lot of concurrent medication (e.g., analgesics). This could explain the increase in serum creatinine levels during hospitalization.
Furthermore, a change in pump parameters, such as an increase in motor power and a decrease in pump flow, are indirect signs of pump thrombosis. In our study cohort motor power and pump flow were significantly elevated at admission. Rise in motor power could be a consequence of a pump thrombosis, but a rise in pump flow cannot be explained by a thrombotic event. Pump parameters of six patients were missing at the time of hospital discharge, confounding the results.
With the results of this study we could show, that 50% of the patients, treated with argatroban for pump thrombosis, showed a successful resolution of the thrombus and 30.8% were free of a thrombotic event for a long post-operative follow-up period (80–1,504 days after discharge). Out of the patient cohort, which needed a VAD exchange due to insufficient argatroban therapy, only 23.1% were free of thrombosis for a long term (80–1,504 days after discharge).
There is a dearth of literature on argatroban therapy for pump thrombosis making it challenging to provide a comprehensive comparison of the data from this study with previous literature. In a case series with four patients Badiye et al. could show a successful resolution of the thrombus in three patients (28). Stulak et al. reported a successful medical treatment of pump thrombosis with tissue plasminogen activator, heparin or heparin and GP IIb/IIIa inhibitor of 48% (31). Only 21% patients of the study did not display re-thrombosis for 30 days (31). Other studies show an even lower rate of success for conservative therapy in pump thrombosis. Eptifibatide has been shown to successfully resolve pump thrombus in 17–22% (26). New data on tissue plasminogen activator is promising, indicating successful treatment in pump thrombosis of 66% (32).
The most common complications of a thrombolytic therapy are bleeding events. Hemorrhagic stroke is an especially debilitating adverse complication of thrombosis. In our study group 23.1% of all patients displayed bleeding events after argatroban therapy. Hemorrhagic strokes were not observed in our patient cohort and the most commonly observed complications were gastrointestinal bleedings, 11.5% of all patients died during the follow up period, but none because of bleeding events. In studies with eptifibatide authors reported a bleeding event rate of 64.7% and a rate of intraparenchymal hemorrhage as cause of death of 41% (26). Schrage et al. reported a rate of bleeding events for tissue plasminogen activator of 6%, but it has to be considered that the study cohort consisted of only nine patients (32). In another study medical treatment of pump thrombosis with tissue plasminogen activator, heparin or heparin and GP IIb/IIIa inhibitor has shown to cause hemorrhage in 21% and death in 10% patients (31). Therefore, in comparison with the other available conservative therapeutic agents argatroban seems to be a promising option.
Without device explantation diagnosis of pump thrombosis can only be suspected and not verified. Additionally different studies on conservative treatment of pump thrombosis were performed on different VAD systems. In our study cohort most of the patients were on a HVAD system, but patients with HM II, HM3, HA5 and MVAD have also been included. Whether there is a direct correlation between a particular pump type and pump thrombosis has not yet been established (32). Patient specific characteristics also play an important role in development of pump thrombosis (33). This makes the comparison of the different studies difficult and subject to potential bias.
Limitations of the study
This study has some limitations. The data was retrospectively collected and analyzed and therefore is subject to the limitations associated with retrospective studies. All exchange procedures were performed at one institution and therefore may be affected by institutional experience. The study cohort with 26 patients is rather small, and could lead to an under- or overestimation of our results.
Conclusions
In comparison with the other available conservative therapeutic agents argatroban appears to be a promising and safe option for patients with VAD-thrombosis. Further studies on a bigger study population are therefore required to evaluate the effectiveness and safety of argatroban administration as therapeutic agent in pump thrombosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: JD Schmitto and G Dogan are consultants of Medtronic Corporation. JD Schmitto receives consultation fees and financial grants from Abbott Laboratories. The other authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the ethics committee of the Hannover Medical School (Application number = 2204:2014).
References
- Transplantation TISfHL. ISHLT heart transplantation 2013 adult heart transplants. % of Patients bridged with mechanical circulatory support by year and device type. 2013.
- Hanke JS, Rojas SV, Martens A, et al. Minimally invasive left ventricular assist device implantation with outflow graft anastomosis to the innominate artery. J Thorac Cardiovasc Surg 2015;149:e69-70. [Crossref] [PubMed]
- Schmitto JD, Hanke JS, Rojas S, et al. Circulatory support exceeding five years with a continuous-flow left ventricular assist device for advanced heart failure patients. J Cardiothorac Surg 2015;10:107. [Crossref] [PubMed]
- Schmitto JD, Rojas SV, Haverich A. Left Ventricular Assist Devices for Advanced Heart Failure. N Engl J Med 2017;376:1894. [PubMed]
- Schmitto JD, Zimpfer D, Fiane AE, et al. Long-term support of patients receiving a left ventricular assist device for advanced heart failure: a follow-up analysis of the Registry to Evaluate the HeartWare Left Ventricular Assist System. Eur J Cardiothorac Surg 2016;50:834-8. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Past, present, and future of minimally invasive mitral valve surgery. J Heart Valve Dis 2011;20:493-8. [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [Crossref] [PubMed]
- Haberl T, Riebandt J, Mahr S, et al. Viennese approach to minimize the invasiveness of ventricular assist device implantationdagger. Eur J Cardiothorac Surg 2014;46:991-6; discussion 996. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Avsar M, et al. Minimally-invasive LVAD Implantation: State of the Art. Curr Cardiol Rev 2015;11:246-51. [Crossref] [PubMed]
- Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012;143:511-3. [Crossref] [PubMed]
- Schmitto JD, Avsar M, Haverich A. Increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:1463-4. [Crossref] [PubMed]
- Krabatsch T, Netuka I, Schmitto JD, et al. Heartmate 3 fully magnetically levitated left ventricular assist device for the treatment of advanced heart failure -1 year results from the Ce mark trial. J Cardiothorac Surg 2017;12:23. [Crossref] [PubMed]
- Netuka I, Sood P, Pya Y, et al. Fully Magnetically Levitated Left Ventricular Assist System for Treating Advanced HF: A Multicenter Study. J Am Coll Cardiol 2015;66:2579-89. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant 2014;33:555-64. [Crossref] [PubMed]
- Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant 2013;32:667-70. [Crossref] [PubMed]
- Nguyen AB, Uriel N, Adatya S. New Challenges in the Treatment of Patients With Left Ventricular Support: LVAD Thrombosis. Curr Heart Fail Rep 2016;13:302-9. [Crossref] [PubMed]
- Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation 2012;125:3038-47. [Crossref] [PubMed]
- Stainback RF, Estep JD, Agler DA, et al. Echocardiography in the management of patients with left ventricular assist devices: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2015;28:853-909. [Crossref] [PubMed]
- Mishkin JD, Enriquez JR, Meyer DM, et al. Utilization of cardiac computed tomography angiography for the diagnosis of left ventricular assist device thrombosis. Circ Heart Fail 2012;5:e27-9. [Crossref] [PubMed]
- Tschirkov A, Nikolov D, Tasheva I, et al. Successful fibrinolysis after acute left ventricular assist device thrombosis. J Heart Lung Transplant 2007;26:553-5. [Crossref] [PubMed]
- Bartoli CR, Ailawadi G, Kern JA. Diagnosis, nonsurgical management, and prevention of LVAD thrombosis. J Card Surg 2014;29:83-94. [Crossref] [PubMed]
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40. [Crossref] [PubMed]
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157-87. [Crossref] [PubMed]
- Blais DM, Sun B, Vesco P, et al. Profound thrombocytopenia with glycoprotein IIb/IIIa inhibitors plus heparin for pump thrombus. J Heart Lung Transplant 2008;27:1361-2. [Crossref] [PubMed]
- Al-Quthami AH, Jumean M, Kociol R, et al. Eptifibatide for the treatment of HeartMate II left ventricular assist device thrombosis. Circ Heart Fail 2012;5:e68-70. [Crossref] [PubMed]
- Tellor BR, Smith JR, Prasad SM, et al. The use of eptifibatide for suspected pump thrombus or thrombosis in patients with left ventricular assist devices. J Heart Lung Transplant 2014;33:94-101. [Crossref] [PubMed]
- Swan SK, Hursting MJ. The pharmacokinetics and pharmacodynamics of argatroban: effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy 2000;20:318-29. [Crossref] [PubMed]
- Badiye A, Hernandez GA, Chaparro S. Argatroban as novel therapy for suspected thrombosis in patients with continuous-flow left ventricle assist device and hemolysis. ASAIO J 2014;60:361-5. [Crossref] [PubMed]
- Moazami N, Milano CA, John R, et al. Pump replacement for left ventricular assist device failure can be done safely and is associated with low mortality. Ann Thorac Surg 2013;95:500-5. [Crossref] [PubMed]
- Stulak JM, Cowger J, Haft JW, et al. Device exchange after primary left ventricular assist device implantation: indications and outcomes. Ann Thorac Surg 2013;95:1262-7; discussion 1267-8. [Crossref] [PubMed]
- Stulak JM, Dunlay SM, Sharma S, et al. Treatment of device thrombus in the HeartWare HVAD: Success and outcomes depend significantly on the initial treatment strategy. J Heart Lung Transplant 2015;34:1535-41. [Crossref] [PubMed]
- Schrage B, Grahn H, Wagner FM, et al. Effective treatment with a new protocol using tissue-type plasminogen activator thrombolysis for pump thrombosis with the HVAD device. Eur Heart J Acute Cardiovasc Care 2017.2048872616688418. [PubMed]
- Blitz A.. Pump thrombosis-A riddle wrapped in a mystery inside an enigma. Ann Cardiothorac Surg 2014;3:450-71. [PubMed]