Rare causes of hyperbilirubinemia after lung transplantation: our experience at a single center
Introduction
Bilirubin is the catabolic end-product of heme metabolism which originates from the degradation of erythrocyte hemoglobin in the reticuloendothelial system, inefficient erythropoiesis in bone marrow, and degradation of other heme proteins (1). The level of serum total bilirubin is widely used to identify hepatobiliary function and hemolytic diseases (2). The etiology of hyperbilirubinemia is multifactorial and includes advanced liver disease, sepsis, and bleeding (3,4). Thus, clearly identifying the cause of persistent hyperbilirubinemia is essential to guide subsequent treatment (3,4).
Lung transplantation has become an established treatment option for patients with end-stage lung disease, and the number of lung transplantations has steadily increased (5). As lung transplantation has become more widespread, several complications after lung transplantation have been reported (6,7). Hyperbilirubinemia after organ transplantation has been reported (8-10), and we have encountered rare causes of hyperbilirubinemia after lung transplantation at our center. Previous research reported that hyperbilirubinemia is common among patients with pulmonary hypertension and that these patients have greater mortality during heart–lung transplantation (11). However, most cases of hyperbilirubinemia were associated with transplantation of organs other than the lungs.
In this study, we investigated the cause, frequency, prognosis and clinical characteristics of unexpected and rare causes of hyperbilirubinemia after lung transplantation.
Methods
Study design
This study was a retrospective case series of patients who were developed hyperbilirubinemia after lung transplantation. Between December 22, 2010 and January 01, 2016, 116 patients underwent lung transplantation at Severance Hospital and Gangnam Severance Hospital in South Korea.
All donor lungs were transplanted from patients after brain death and preserved using low-potassium dextran solution (Perfadex®; Duraent Biologicals, Hyderabad, India). During lung transplantation surgery, extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass was applied to all patients for cardiopulmonary support. All patients were received corticosteroids for immunosuppression at the time of transplantation surgery and maintained on triple immunosuppression therapy including prednisolone, tacrolimus, and mycophenolate mofetil (MMF) after transplantation.
Data collection and definition
The normal range of serum total bilirubin at our center is between 0.4 and 1.5 mg/dL. We defined hyperbilirubinemia as total bilirubin level exceeding 5 mg/dL for at least 3 days after lung transplantation. We defined common cause of hyperbilirubinemia that included drug toxicity, sepsis, biliary tract stone, bleeding and other clinically easily predicted causes. Patient data such as demographic, laboratory, mortality, medications, imaging, and other data were collected from the hospital’s electronic medical records.
Statistical analysis
Data were expressed as median plus range or interquartile range. Statistical analyses were performed with SPSS version 23 statistical software. Differences between rare causes of hyperbilirubinemia group and common causes of hyperbilirubinemia group were compared using the Mann Whitney U test. P<0.05 were considered statistically significant.
Results
A total of 116 patients received lung transplantation, and 33 (28.4%) lung transplant recipients developed hyperbilirubinemia during the study period (Table 1). The leading cause of lung transplantation was idiopathic pulmonary fibrosis (48.3%), followed by bronchiolitis obliterans after stem cell transplantation (12.1%) and interstitial lung disease related to connective tissue disease (10.3%). Ten patients out of 116 patients had chronic liver disease. Among them, four patients had mild fatty liver disease, four patients had well controlled chronic hepatitis B, one patient had alcoholic liver disease, and one had liver failure. The patient with liver failure patient underwent simultaneous lung and liver transplantation.
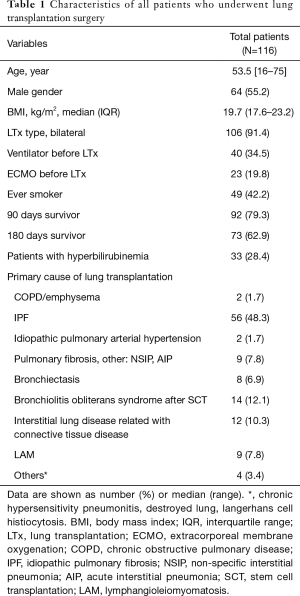
Full table
Among the 33 patients with hyperbilirubinemia, 24 patients had common causes of hyperbilirubinemia such as drug toxicity, biliary tract stone, sepsis, bleeding, and liver failure after lung transplantation with liver transplantation. Nine patients had unexpected, rare causes of hyperbilirubinemia including hemophagocytic lymphohistiocytosis (HLH), thrombotic microangiopathy (TMA), and ischemic cholangiopathy.
The baseline characteristics of patients with hyperbilirubinemia after lung transplantation are described in Table 2. The most common cause of hyperbilirubinemia was sepsis (N=11, 33.3%). Patients who developed hyperbilirubinemia had a poor prognosis, and the recovery rate of hyperbilirubinemia was low (N=5, 15.2%). Causes of normalized hyperbilirubinemia were bleeding, drugs, and biliary tract stones. Four of the five patients who recovered from hyperbilirubinemia were still alive at the 180-day follow-up, and the remaining 29 patients with hyperbilirubinemia expired within 180 days after lung transplantation. Sepsis was the leading cause of death (N=16), followed by liver failure due to ischemic cholangiopathy (N=4), TMA (N=3), HLH (N=2), bleeding (N=2) and sudden cardiac arrest (N=2). Median age, male sex, and median body mass index did not differ according to cause of hyperbilirubinemia. However, the median onset time of hyperbilirubinemia after lung transplantation in cases with rare causes was relatively late (88 vs. 6.5 days, P<0.001) compared to cases with common causes. Three patients among those with hyperbilirubinemia had liver disease and no distinct types were noted.
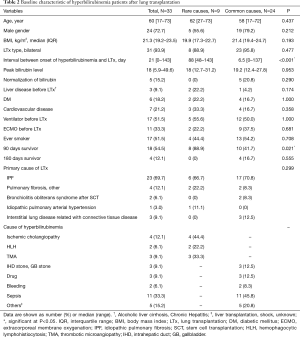
Full table
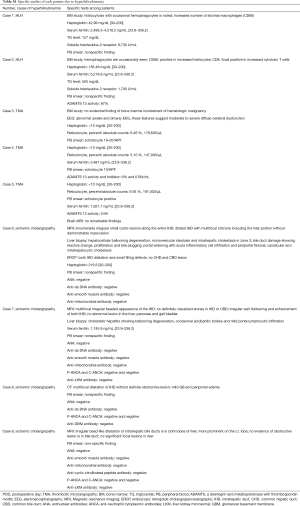
The demographic characteristics of the 9 patients with rare causes of hyperbilirubinemia are presented in Table 3. Two patients had HLH, and both were intubated and mechanically ventilated before transplantation. Patients underwent imaging using abdominal ultrasonography, abdominal computed tomography (CT), or magnetic resonance cholangiopancreatography (MRCP) after developing hyperbilirubinemia. However, imaging studies did not reveal abnormalities in the biliary tract or liver parenchyma. Patients had hyperbilirubinemia with ongoing pancytopenia of unknown origin. Various laboratory tests were performed (Table S1); however, the reason for pancytopenia was not determined. Finally, bone marrow aspiration and biopsy were conducted. HLH was diagnosed based on the clinical findings, laboratory findings, and bone marrow biopsy results.
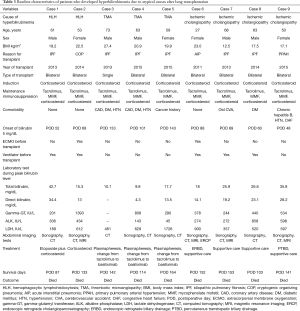
Full table
Full table
Three patients were diagnosed with TMA. None were intubated or mechanically ventilated before transplantation. All underwent abdominal imaging with ultrasonography and CT upon detection of hyperbilirubinemia. However, imaging did not reveal abnormalities in the biliary tract or liver parenchyma. We conducted a peripheral blood smear due to thrombocytopenia and discovered schistocytes. Additionally, the haptoglobulin level was low, and the reticulocyte level was high, which suggested hemolytic anemia. One patient underwent a bone marrow biopsy, which showed nonspecific findings. To evaluate microangiopathic hemolytic anemia (MAHA), ADAMTS13 activity was evaluated in two patients and found to be normal or only mildly decreased (Table S1). We supposed that hyperbilirubinemia was caused by TMA, which might be related to the use of tacrolimus.
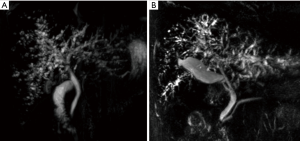
Four patients were diagnosed with ischemic cholangiopathy. Three were intubated and mechanically ventilated before transplantation. Patients underwent abdominal imaging with several modalities including ultrasonography, CT, and MRCP. Imaging studies revealed features of ischemic cholangiopathy and no obstruction of the biliary tract (Figure 1). Case 6 was diagnosed after undergoing endoscopic retrograde cholangiopancreatography (ERCP), liver biopsy and imaging. The ERCP revealed small filling defects and dilatation of both intrahepatic ducts. Liver biopsy in case 6 revealed intrahepatic cholestasis and portal widening with acute inflammatory cell infiltration. The result of the liver biopsy in case 7 revealed the development of cholestatic hepatitis. Cases 8 and 9 were diagnosed without liver biopsy because these patients had very similar findings with regard to laboratory studies, imaging studies, and clinical characteristics as cases 6 and 7.
The time interval between transplantation and hyperbilirubinemia was approximately 2 months in patients with HLH and ischemic cholangiopathy, and approximately 4 months in patients with TMA (Table 3).
The two patients diagnosed with HLH were treated with etoposide plus steroid or steroid alone, and the three patients who developed TMA had their treatment changed to basiliximab from tacrolimus with plasmapheresis. The four patients with ischemic cholangiopathy received supportive treatment including endoscopic retrograde biliary drainage or percutaneous transhepatic biliary drainage. However, all nine patients with hyperbilirubinemia of rare causes died due to progression of HLH, TMA or ischemic cholangiopathy during long term intensive care unit treatment (Table 3).
Discussion
In the present study, we found that hyperbilirubinemia occurred at a rate of 28.4% (33/116) in patients who received lung transplants at our institution, and the rate of hyperbilirubinemia due to rare causes such as HLH, TMA, and ischemic cholangiopathy was 7.8% (9/116) during the study period.
We further found that most causes of severe hyperbilirubinemia were not hepatic but systemic problems and associated with poor prognosis. In our institution, the most common cause of 1-year mortality was infection (58.3%), followed by cardiac arrest (12.5%) and TMA (12.5%). Some patients died due to HLH, ischemic cholangiopathy, or bleeding. Except sudden cardiac arrest, most causes of mortality could induce hyperbilirubinemia (12).
HLH is a potentially life-threatening hyperinflammatory syndrome that is divided into primary and secondary (or acquired) HLH. Our cases were secondary HLH. Acquired HLH without a genetic cause develops due to infections, autoimmune diseases, malignancies or other stimuli. A number of clinical symptoms can be observed in patients with HLH including persistent fever, multiple organ involvement, and laboratory abnormalities including cytopenia, increased serum ferritin level, abnormal liver function tests (aspartate transaminase, alanine aminotransaminase, gamma-glutamyl transferase, bilirubin, lactate dehydrogenase), and abnormal coagulation parameters. High-dose corticosteroid, etoposide, and cyclosporine have been suggested as treatments for primary HLH. However, a treatment regimen for acquired HLH does not yet exist, and the prognosis of acquired HLH is poorer than that of primary HLH (13). The poor prognosis of our cases with acquired HLH after lung transplantation is consistent with previous reports (14,15). In patients who undergo lung transplantation, the immune system is altered by the use of immunosuppressive drugs. Therefore, these patients are at high risk of opportunistic infection and sepsis. We speculate that infection and severe inflammation in our cases were associated with HLH after lung transplantation. Although the pathogenesis of acquired HLH remains unclear, several studies suggest that HLH is associated with inflammation (16-18). Furthermore, in many patients, HLH may be accompanied by hepatitis, because HLH induces excessive inflammation and tissue destruction (19). Therefore, HLH should be considered in the differential diagnosis of hyperbilirubinemia after lung transplantation when the origin of hyperbilirubinemia is unknown.
TMA induced by various causes is characterized by MAHA, thrombocytopenia, microvascular thrombosis, and organ injury (20). Several drugs associated with TMA have been reported (20,21). These drugs mediated TMA through immune or toxic dose-duration-related reactions (20). In an analysis of 387 articles, Al-Nouri et al. reported that 22 drugs had definite evidence of association with TMA, three of which (quinine, cyclosporine, and tacrolimus) accounted for approximately 58 percent of drug-induced TMA cases (21). We speculate that the TMA cases in our study were associated with tacrolimus, because all patients developed hyperbilirubinemia after at least 3 months of immunosuppressive therapy, and TMA temporarily improved after stopping the use of tacrolimus with plasma exchange. Furthermore, we could not find other causes of TMA. ADAMTS13 activity and complement level support this diagnosis. MAHA, which is caused by TMA, increases the indirect bilirubin level by destroying red blood cells. Therefore, TMA should be considered in the differential diagnosis of hyperbilirubinemia of unknown origin after lung transplantation, because urgent management in suspected TMA is important (22).
Ischemic cholangiopathy is induced by impaired blood supply, especially the peribiliary vascular plexus from hepatic arteries (23). Ischemic cholangiopathy can have various causes including liver transplantation, vascular injury during surgery, chemotherapy, biliary ischemia in hereditary hemorrhagic telangiectasia, or secondary sclerosing cholangitis in critically ill patients (23,24). Patients with ischemic cholangiopathy present with clinical features including jaundice, pruritus, and dark urine. Although there are several radiological features of primary sclerosing cholangitis, abdominal ultrasonography or CT can reveal normal findings. Therefore, the diagnosis of ischemic cholangiopathy generally requires magnetic resonance imaging (MRI) or cholangiography (23,25). Liver transplantation is the most common cause of ischemic cholangiopathy (23); however, ischemic cholangiopathy after lung transplantation has not been previously reported. We speculate that our cases were associated with prolonged hypoxic damage due to ventilator care before transplantation and septic shock after transplantation. In view of prolonged ischemia, several papers have reported on the relationship between critical illness and ischemic cholangiopathy (24,26,27). Abbasi et al. reported that longer duration of ECMO and ECMO-related complications were associated with the development of cholestasis in neonates (28). In our study, only one of our patients with ischemic cholangiopathy had experienced ECMO before lung transplantation. Seventeen patients with hyperbilirubinemia weaned the ECMO in the intensive care unit (ICU) after lung transplantation. One out of 17 patients was diagnosed with ischemic cholangiopathy. There was no association between delayed ECMO weaning or duration of ECMO use and cause of hyperbilirubinemia. Therefore, the relationship between ECMO and ischemic cholangiopathy was unclear in our study. The optimal treatment of ischemic cholangiopathy has not yet been established (23). Despite efforts including endoscopic retrograde biliary drainage and percutaneous transhepatic biliary drainage, all our patients died. Taken together, ischemic cholangiopathy should also be considered in the differential diagnosis of hyperbilirubinemia when patients have been exposed to shock or have findings of obstructive jaundice without a definite obstruction on abdominal ultrasonography or CT.
Although causes of hyperbilirubinemia can vary widely (3,4), only few cases of hyperbilirubinemia with rare causes after lung transplantation have been reported (14,15,29-31). Furthermore, we did not find any reports on the association of lung transplantation with ischemic cholangiopathy. Patients with rare causes of hyperbilirubinemia after lung transplantation had a poor prognosis; therefore, early evaluation and management of hyperbilirubinemia are essential to improving prognosis. Several imaging studies including abdominal ultrasonography, CT, and MRI; clinical manifestations; time interval between transplantation and hyperbilirubinemia detection; and various laboratory tests may be helpful for the differential diagnosis of causes of hyperbilirubinemia.
This study has several limitations. First, the sample size is relatively small. Second, this study is a retrospective analysis and a single-center experience. Third, we cannot rule out the effects of cardiopulmonary support because we used ECMO or cardiopulmonary bypass during all operations. Finally, patients who developed hyperbilirubinemia may have complex, multifactorial causes of hyperbilirubinemia and we were unable to account for all of these due to the retrospective nature of this study. Additional prospective and multicenter studies are needed to assess the incidence, causes, prognosis, and risk factors of hyperbilirubinemia after lung transplantation.
Conclusions
Causes of hyperbilirubinemia after lung transplantation are varied, and the prognosis of patients with rare causes of hyperbilirubinemia was poor. Therefore, early evaluation and management of hyperbilirubinemia may be necessary if it develops after lung transplantation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Institutional Review Board (IRB) of Severance Hospital (IRB number: 2013-0522-019). Informed consent was waived by the IRB because of the study’s retrospective nature.
References
- Sticova E, Jirsa M. New insights in bilirubin metabolism and their clinical implications. World J Gastroenterol 2013;19:6398-407. [Crossref] [PubMed]
- Horsfall LJ, Rait G, Walters K, et al. Serum bilirubin and risk of respiratory disease and death. JAMA 2011;305:691-7. [Crossref] [PubMed]
- Reisman Y, Gips CH, Lavelle SM, et al. Clinical presentation of (subclinical) jaundice--the Euricterus project in The Netherlands. United Dutch Hospitals and Euricterus Project Management Group. Hepatogastroenterology 1996;43:1190-5. [PubMed]
- Pratt DS, Kaplan MM. Evaluation of Abnormal Liver-Enzyme Results in Asymptomatic Patients. N Engl J Med 2000;342:1266-71. [Crossref] [PubMed]
- Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant 2012;31:1073-86. [Crossref] [PubMed]
- Timrott K, Vondran FW, Kleine M, et al. The impact of abdominal complications on the outcome after thoracic transplantation--a single center experience. Langenbecks Arch Surg 2014;399:789-93. [Crossref] [PubMed]
- Grass F, Schafer M, Cristaudi A, et al. Incidence and Risk Factors of Abdominal Complications After Lung Transplantation. World J Surg 2015;39:2274-81. [Crossref] [PubMed]
- Hsu RB, Lin FY, Chen RJ, et al. Incidence, risk factors, and prognosis of postoperative hyperbilirubinemia after heart transplantation. Eur J Cardiothorac Surg 2007;32:917-22. [Crossref] [PubMed]
- Barba P, Martino R, Perez-Simon JA, et al. Incidence, characteristics and risk factors of marked hyperbilirubinemia after allogeneic hematopoietic cell transplantation with reduced-intensity conditioning. Bone Marrow Transplant 2012;47:1343-9. [Crossref] [PubMed]
- Gates LK Jr, Wiesner RH, Krom RA, et al. Etiology and incidence of unconjugated hyperbilirubinemia after orthotopic liver transplantation. Am J Gastroenterol 1994;89:1541-3. [PubMed]
- Kramer MR, Marshall SE, Tiroke A, et al. Clinical significance of hyperbilirubinemia in patients with pulmonary hypertension undergoing heart-lung transplantation. J Heart Lung Transplant 1991;10:317-21. [PubMed]
- Lee SH, Park MS, Song JH, et al. Perioperative factors associated with 1-year mortality after lung transplantation: a single-center experience in Korea. J Thorac Dis 2017;9:4006-16. [Crossref] [PubMed]
- Janka GE, Lehmberg K. Hemophagocytic syndromes--an update. Blood Rev 2014;28:135-42. [Crossref] [PubMed]
- Oto T, Snell GI, Goto K, et al. Hemophagocytic syndrome: a rare but specific complication of lung transplantation. J Thorac Cardiovasc Surg 2010;140:e58-9. [Crossref] [PubMed]
- Diaz-Guzman E, Dong B, Hobbs SB, et al. Hemophagocytic lymphohistiocytosis after lung transplant: report of 2 cases and a literature review. Exp Clin Transplant 2011;9:217-22. [PubMed]
- Sinha S, Mishra SK, Sharma S, et al. Polymorphisms of TNF-enhancer and gene for FcgammaRIIa correlate with the severity of falciparum malaria in the ethnically diverse Indian population. Malar J 2008;7:13. [Crossref] [PubMed]
- Poggi A, Costa P, Tomasello E, et al. IL-12-induced up-regulation of NKRP1A expression in human NK cells and consequent NKRP1A-mediated down-regulation of NK cell activation. Eur J Immunol 1998;28:1611-6. [Crossref] [PubMed]
- Xu A, Bellamy AR, Taylor JA. Immobilization of the early secretory pathway by a virus glycoprotein that binds to microtubules. Embo J 2000;19:6465-74. [Crossref] [PubMed]
- Jordan MB, Allen CE, Weitzman S, et al. How I treat hemophagocytic lymphohistiocytosis. Blood 2011;118:4041-52. [Crossref] [PubMed]
- George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med 2014;371:654-66. [Crossref] [PubMed]
- Al-Nouri ZL, Reese JA, Terrell DR, et al. Drug-induced thrombotic microangiopathy: a systematic review of published reports. Blood 2015;125:616-8. [Crossref] [PubMed]
- Barbour T, Johnson S, Cohney S, et al. Thrombotic microangiopathy and associated renal disorders. Nephrol Dial Transplant 2012;27:2673-85. [Crossref] [PubMed]
- Deltenre P, Valla DC. Ischemic cholangiopathy. J Hepatol 2006;44:806-17. [Crossref] [PubMed]
- Horvatits T, Trauner M, Fuhrmann V. Hypoxic liver injury and cholestasis in critically ill patients. Curr Opin Crit Care 2013;19:128-32. [Crossref] [PubMed]
- Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660-78. [Crossref] [PubMed]
- Cohen L, Angot E, Goria O, et al. Ischemic cholangiopathy induced by extended burns. Ann Pathol 2013;33:113-6. [Crossref] [PubMed]
- Gelbmann CM, Rummele P, Wimmer M, et al. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol 2007;102:1221-9. [Crossref] [PubMed]
- Abbasi S, Stewart DL, Radmacher P, et al. Natural course of cholestasis in neonates on extracorporeal membrane oxygenation (ECMO): 10-year experience at a single institution. Asaio J 2008;54:436-8. [Crossref] [PubMed]
- Go O, Naqvi A, Tan A, et al. The spectrum of thrombotic thrombocytopenic purpura: a clinicopathologic demonstration of tacrolimus-induced thrombotic thrombocytopenic purpura in a lung transplant patient. South Med J 2008;101:744-7. [Crossref] [PubMed]
- Roberts P, Follette D, Allen R, et al. Cyclosporine A-associated thrombotic thrombocytopenic purpura following lung transplantation. Transplant Proc 1998;30:1512-3. [Crossref] [PubMed]
- Lovric S, Kielstein JT, Kayser D, et al. Combination of everolimus with calcineurin inhibitor medication resulted in post-transplant haemolytic uraemic syndrome in lung transplant recipients--a case series. Nephrol Dial Transplant 2011;26:3032-8. [Crossref] [PubMed]


