Photodynamic therapy versus endoscopic submucosal dissection for management of patients with early esophageal neoplasia: a retrospective study
Introduction
Esophageal cancer is one of the most common worldwide malignant tumours. The 5-year survival rate for highly aggressive esophageal squamous cell carcinoma is less than 20% (1). Previously, the treatment for early esophageal neoplasia was esophagectomy. Nowadays, it can be cured by endoscopic therapies such as endoscopic submucosal dissection (ESD), cryotherapy thermal ablation and photodynamic therapy (PDT). These lesions are prominent well-demarcated tumours appearing as irregular dyschromia areas of mucosa (2,3).
PDT as known is based on the photochemical reaction produced by photosensitizer and specific wavelengths of visible light which has the potential to cure early esophageal neoplasia (4,5). Overholt et al.’s (6) study indicated that PDT was an effective mean for eliminating the high-grade dysplasia of patients with Barrett’s esophagus and could reduce the incidence of esophageal adenocarcinoma. PDT can be operated repeatedly without producing any resistance or cumulative toxicity, so it is relatively a new therapeutic modality with distinct advantages (7). In Addition, PDT can be used in combination with other therapies. Even if it did not achieve the desired therapeutic effect, we may consider other invasive means of intervention with the primary lesion.
Hosokawa and colleagues have successfully applied ESD for resection of early gastric cancer for the first time (8). With the help of minimally invasive endoscopic resection techniques, we can achieve large bloc resection for early esophageal cancer. Although ESD is difficult as far as technology, it has been shown to be an effective and relatively safe treatment for the early esophageal squamous cell carcinoma (9-11).
In Japan, although PDT has been approved as a curative treatment for superficial esophageal cancer, it lost the popularity due to the dramatic spreading of ESD (12). Until now comparative studies focusing on ESD and PTD have not been yet reported. So the aim of this study is to analyze the difference between ESD and PDT for management of patients with early esophageal cancer regarding the postoperative outcomes and survival.
Methods
From January 2014 to March 2015, patients at high risk of esophageal neoplasia received careful screening with electronic gastroscopy (GIF-H260J; Olympus Co. Ltd., Japan). Lugol staining (concentration of 1.5%) and narrow-band imaging (NBI) were performed on all the patients to determine suitability for resection or irradiation range. Low-grade dysplasia, high-grade dysplasia, and carcinoma confined to the mucosal layer are all defined as “early” esophageal neoplasia. Depth of invasion and regional lymph node were determined by Endoscopic ultrasonography (EUS). All patients underwent chest and abdominal computed tomography (CT) scan to exclude the presence of metastasis. Positron emission tomography—computed tomography (PET-CT) was applied to patients whose biopsy had revealed carcinoma or those with dysplasia with suspicious lymph node metastasis detected by EUS or CT. Once the depth of invasion the tumor was beyond the mucosal layer or there was existence of regional lymph node and distant metastasis, these patients would be excluded from ESD or PDT. The study was approved by Ethics Committee of Henan Cancer Hospital. Written informed consent was waived because of the nature of retrospective study.
ESD procedure
Regarding ESD procedure, we used lugol spray and NBI to demarcate the boundaries of the lesion. Several spots were marked at 2–3 mm away from the margin of the esophageal neoplasia by using argon plasma coagulation (ERBE, Tuebingen, Germany) to ensure a clear safety margin. Then, 3–5 mL glycerol solution plus indigo-carmine with 0.0025% epinephrine was injected into the submucosal layer using a 23-gauge disposable injector to raise the lesion. A circumferential incision was made initially, followed by submucosal dissection with the IT-knife 2 (KD-610L; Olympus Co. Ltd., Tokyo, Japan). Hemostatic forceps (FD-410LR; Olympus) was used in a soft coagulation mode (60-W output) to control bleeding.
PDT procedure
The domestic photosensitizer hematoporphyrin derivative injection (commodity name: Hipofin, 20 mL: 0.1 g) produced by Chongqing Huading biological pharmaceutical limited company was used to the PDT of superficial esophageal carcinoma. The administrated dose of photosensitizer was 2 mg/kg. 5% glucose solution should be infused intravenously for one hour with the photosensitizer by the proportion of 2.5mg/mL. Then PDT was performed 24–48 hours later. The laser light was delivered circumferentially using cylindrical diffusing quartz fiber (Biolitec CD603-20 C, diffuser of 2 cm length) passing through the channel of the endoscope while the patient is under general anesthesia. As recommended, after calibration of the fiber, a total light dose of 250–300 J/cm was administered at a non-thermal fluency rate during a treatment time of 600 s at a power setting of 1W provided by a 630 nm diode laser source (Diomed Ltd., Cambridge, UK). And the dose received by the lesion was 130 J/cm2. After 72–96 hours, the necrosis was removed partially and PDT was then repeated. Patients were discharged 2 days later with special recommendation for avoiding all weavers light during 3 weeks. Dilatation was performed if dysphagia was present.
Follow-up
All the patients received regular endoscopic follow-us every 1 month during the first 6 months, every 2 months for the next 6 months of the first year, then, every 3 months during the second year.
Data acquisition
Data was analyzed by SPSS version 17.0 (SPSS, IBM, Chicago, IL, USA). Continuous data was expressed as the mean ± SD and was analyzed by the unpaired t-test. Categorical variables were presented as frequency (%) and were analyzed by the χ2 test. The Kaplan—Meier method was used to test the associated risk factors for the time of recurrence and metastasis. Comparisons between groups were calculated by log-rank test. P<0.05 was considered significant.
Results
Sixty-six patients (34 males and 32 females) were included in our department of thoracic surgery for treatment of early esophageal neoplasia. Among them, 30 were referred for PDT, and other 36 patients accepted ESD (Table 1). Circumferential extension of tumor was present in less than half of the patients (29 patients). Fifty percent to 75% of the circumference was involved by the tumour in 22 patients, and more than 75% in 15 patients. Regarding the pathology, 29 patients had high-grade dysplasia, 33 patients had Squamous cell carcinoma and 4 were having Adenocarcinoma. No significant differences were observed in sex, age, smoking, drinking, pathology, and circumferential extension of tumor (P>0.05). Also, there was no significant difference in hospital length of stay, presence of hydrothorax, fever, and pain. But there was a significant statistical difference in operative time, cost, bleeding, perforation, and stricture (P<0.05) (Table 2). Using the Kaplan–Meier method to compare between both groups regarding disease-free survival, there were no significant differences between ESD and PDT (P>0.05) (Table 3, Figure 1).
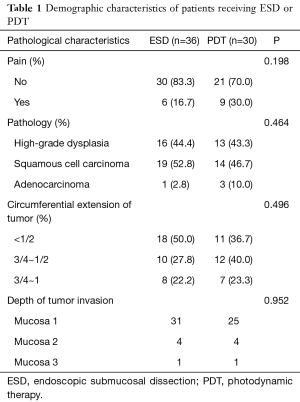
Full table
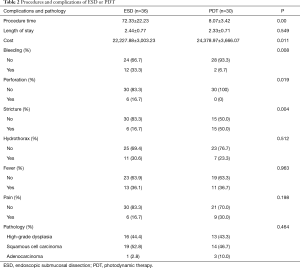
Full table
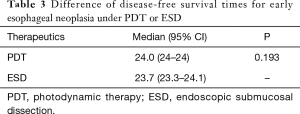
Full table
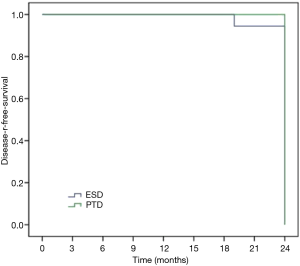
Discussion
Upper gastrointestinal endoscopy is a valuable tool for the diagnosis, staging and treatment of early-stage esophageal neoplasia. Superficial lesions can be found confined to the mucosal layer by endoscopic surveillance. These lesions may present as a prominent well-demarcated tumor. For these lesions, ESD may be most appropriate treatment. It can provide an entire tumor for histopathological examination. Other superficial lesions, present as an irregular dyschromia area of mucosa or may be not well demarcated. PDT may be another means of choice for them. For early esophageal neoplasia, both ESD and PDT have been shown to be safe with long-term survival outcomes which are usually quite near to the surgical operation. They are both suitable for treatment of esophageal precancerous lesion (13,14).
Early esophageal carcinoma includes mucosal and submucosal carcinomas with or without the presence of lymph node metastasis. For these patients without lymph node metastasis, endoscopic resection such as ESD has been suggested as an alternative mean to esophagectomy, and its effect can be matched with surgery (15). Ten years ago, Maunoury’s (16) study provided some promising data for PDT of esophageal carcinomas in selected patients.
PDT is a photosensitive reaction process involving oxygen molecules, which is accompanied by biological effects. Laser irradiation of the specific waves makes the photosensitizer activated and produces single oxygen. It is the oxidation reaction of single oxygen that causes to ischemic necrosis of the tumor tissue (17,18). One study including 103 patients with low-grade dysplasia, high-grade dysplasia, or intramucosal adenocarcinoma revealed the successful rate of ablation was 93%, 78% and 44%, respectively. Savary et al. (19) reviewed 24 cases of early squamous cell carcinomas of the esophagus treated by PDT: 84% of patients were cured after a mean follow-up of 2 years; there were two stenosis and two fistulas.
ESD is widely accepted because of its minimal invasion, low cost, patients’ good tolerance and better quality of life after operation, but its operative time is longer and the incidence of complications is higher (20). Our study shows that the incidence of major complications for ESD is similar to the incidence of Japanese studies for selected early esophageal neoplasia (10,11,21). Lee et al.’s (22) researches show that ESD is a promising local curative treatment option for early esophageal neoplasia. Lian (23) demonstrated that ESD was more promising, but the incidence of perforation and bleeding was higher. In our ESD procedures, the occurrence rate of bleeding and perforation was 33.3%, 16.7% respectively.
To summarize, both ESD and PDT are effective methods of treatment for early esophageal cancers in selected patients. Due to complexity of surgical procedure, the operative time of ESD is much longer than PDT and the bleeding in patients receiving ESD are more than PDT. Zero percent to 3% rates of local recurrence was reported with a R0 ESD in previous studies (10,11,21). In our study, rates of local recurrence are 5.6% (2/36) for ESD, while PDT is 0%. Benign stricture was the most commonly observed complications, the stricture rates of Eunjue et al.’s (24) study was 19.4%, while in our study is 50.0%. Yi’s data suggested that disease-free survival of patients with complete remission was 21.9 months which is similar to ours. PDT is a reasonable palliative treatment option with acceptable complication rates for esophageal cancer and could be performed for therapeutic purposes in cases of early esophageal cancer (24). Of course, PDT can be applied to the advanced esophageal cancer as a palliative means in order to alleviate the symptom of obstruction.
In conclusion, both PDT and ESD are promising local curative treatment options for early esophageal neoplasia. ESD still carries the risks of perforation and bleeding in our study. But PDT is a feasible and safe treatment modality. However, esophageal stricture is an important concern to be aware of in the postoperative follow up of patients undergoing PDT. Although ESD is technically difficult, PDT is much easier. It is currently unclear whether it can be a substitute for surgery in patients with early esophageal cancer. However, PDT is suitable for early esophageal neoplasia with lesion confined to the mucosal layer without regional lymph nodal and distant metastasis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Ethics Committee of Henan Cancer Hospital (No. 2017408). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Barr H, Dix AJ, Kendall C, et al. Review article: the potential role for photodynamic therapy in the management of upper gastrointestinal disease. Aliment Pharmacol Ther 2001;15:311-21. [Crossref] [PubMed]
- Probst A, Aust D, Märkl B, et al. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy 2015;47:113-21. [PubMed]
- Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst 1998;90:889-905. [Crossref] [PubMed]
- Kato H, Horai T, Furuse K, et al. Photodynamic therapy for cancers: a clinical trial of porfimer sodium in Japan. Jpn J Cancer Res 1993;84:1209-14. [Crossref] [PubMed]
- Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc 2005;62:488-98. [Crossref] [PubMed]
- Fisher AM, Murphree AL, Gomer CJ, et al. Clinical and preclinical photodynamic therapy. Lasers Surg Med 1995;17:2-31. [Crossref] [PubMed]
- Ohkuwa M, Hosokawa K, Boku N, et al. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy 2001;33:221-6. [Crossref] [PubMed]
- Fujishiro M, Kodashima S, Goto O, et al. Endoscopic submucosal dissection for esophageal squamous cell neoplasms. Dig Endosc 2009;21:109-15. [Crossref] [PubMed]
- Ishihara R, Iish H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008;68:1066-72. [Crossref] [PubMed]
- Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 2005;3:S67-70. [Crossref] [PubMed]
- Yano T, Hatogai K, Morimoto H, et al. Photodynamic therapy for esophageal cancer. Ann Transl Med 2014;2:29. [PubMed]
- Javaid B. Photodynamic therapy (PDT) for oesophageal dysplasia and early carcinoma with mTHPC (m-Tetrahidroxyphenyl chlorin): a preliminary study. Lasers Med Sci 2002;17:135. [Crossref] [PubMed]
- Tokar JL, Haluszka O, Weinberg DS. Endoscopic therapy of dysplasia and early-stage cancers of the esophagus. Semin Radiat Oncol 2007;17:10-21. [Crossref] [PubMed]
- Inoue H, Minam H, Kaga M, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for esophageal dysplasia and carcinoma. Gastrointest Endosc Clin N Am 2010;20:25-34. v-vi. [Crossref] [PubMed]
- Maunoury V, Mordon S, Bulois P, et al. Photodynamic therapy for early oesophageal cancer. Dig Liver Dis 2005;37:491-5. [Crossref] [PubMed]
- Gross SA, Wolfsen HC. The role of photodynamic therapy in the esophagus. Gastrointest Endosc Clin N Am 2010;20:35-53. vi. [Crossref] [PubMed]
- Wang KK, Lutzke L, Borkenhagen L, et al. Photodynamic therapy for Barrett's esophagus: does light still have a role? Endoscopy 2008;40:1021-5. [Crossref] [PubMed]
- Savary JF, Grosjean P, Fontolliet C, et al. Photodynamic therapy of early squamous cell carcinomas of the esophagus: a review of 31 cases. Endoscopy 1998;30:258-65. [Crossref] [PubMed]
- Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, et al. Endoscopic submucosal dissection for the treatment of neoplastic lesions in the gastrointestinal tract. World J Gastroenterol 2013;19:1953-61. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol 2006;4:688-94. [Crossref] [PubMed]
- Lee CT, Chanq CY, Tai CM, et al. Endoscopic submucosal dissection for early esophageal neoplasia: a single center experience in South Taiwan. J Formos Med Assoc 2012;111:132-9. [Crossref] [PubMed]
- Lian J, Chen S, Zhang Y, et al. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc 2012;76:763-70. [Crossref] [PubMed]
- Yi E, Yang CK, Leem C, et al. Clinical outcome of photodynamic therapy in esophageal squamous cell carcinoma. J Photochem Photobiol B 2014;141:20-5. [Crossref] [PubMed]


