Polymeric films loaded with cisplatin for malignant pleural mesothelioma: a pharmacokinetic study in an ovine model
Introduction
Malignant pleural mesothelioma (MPM) continues to be a distressing tumor due to its aggressive biologic behavior and scanty prognosis. The Third Italian Consensus Conference for Malignant Pleural Mesothelioma held in Bari in January 2015, considered all aspects related to MPM, including epidemiology (1). In 2011, in Italy, the incidence of MPM was 3.49 and 1.25 cases per 100,000 person/years in men and women respectively, with large dissimilarities registered among different geographic regions (1). Despite the fact that all forms of asbestos were banned in the European Union during the past years, the whole amount of mesothelioma cases has not decreased in most European countries (2,3). Moreover, MPM rates in Europe and Japan were estimated to peak in 2020 and 2025, respectively (2). Since mesothelioma is a tumor that appears decades after asbestos exposure, is still a rising and escalating healthcare problem.
MPM is routinely believed to be a neoplasm resistant to any type of therapy, including chemotherapy, radiotherapy and surgery. Until now, the association of these different treatment modalities was able to lead only to a limited benefit in terms of overall survival and symptoms control (4,5). Recent progresses and increasing insight into the biologic features of MPM, including molecular and immunophenotypic characteristics, allowed testing a wide variety of combination chemotherapy and immunotherapy both in preclinical and clinical scenarios (6-9). The interest of the international scientific community focused not only on treatment aspects but also on new diagnostic and prognostic biomarkers that could guide towards targeted therapies (10-12). Locoregional treatment options, consisting in the intrapleural application of anti-tumor agents, have been used in the management of MPM (4-9), usually within a multimodal approach (13). Some years ago, our group concentrated on an innovative drug formulation for the loco-regional treatment of MPM after surgery (7). More specifically, we constructed polymeric films (hyaluronate was the main component) loaded with cisplatin at a concentration of 100 mg/m2. We used an orthotopic rat recurrence model of MPM to compare the hyaluronate cisplatin films (HYALCIS) with the cisplatin solution, both intrapleurally applied. The primary endpoint was the volume of tumor recurrence and the secondary endpoint was the treatment related toxicity. We were able to show that the tumor volume was significantly reduced in the HYALCIS group in comparison to animals treated with cisplatin solution. Moreover, cisplatin was detected in the serum at a concentration six- and seven-fold significantly higher in the HYALCIS group compared to cisplatin solution, and was maintained over time. Although the high levels of cisplatin registered, no significant toxicity related to the different treatments was observed (7). With this regard, the Swiss group coordinated by Prof. Weder, published several papers on the role of intrapleural chemotherapy with cisplatin loaded to the fibrin sealant Vivostat® in the same rat mesothelioma model of tumor recurrence (6,9) and subsequently in pigs (14). The pleural and plasmatic platinum concentrations in the animals treated with the polymeric films were higher than those reported by the Swiss group. As we assumed in the previous paper, we hypothesized that this difference might be due to the pharmacokinetics of the polymeric films, which allows for a gradual cisplatin release and therefore a regular increase in the plasmatic levels of the drug without increasing the systemic toxicity (7). After that, we assessed the potential effect of pemetrexed loaded onto our polymeric films (alone and in association with cisplatin) in this rat mesothelioma model (data not published). The study design was identical and the endpoints were similar to the preceding study, being the primary aim the volume of tumor recurrence and the secondary aim the systemic side effects correlated to the treatment. We observed that animals receiving the polymeric films loaded with cisplatin and pemetrexed had a further decrease of tumor volume with respect to animals treated with HYALCIS, but the difference was not statistically significant (data not published). And as for systemic side effects, the toxicity did not significantly differ among treatment groups. At this point, considering the encouraging results obtained so far, we decided to study the pharmacokinetics of the polymeric films in a large animal model. Therefore, taking into account that sheep are suitable animal models to study the pharmacokinetics of drugs used in man (15), the aim of this study was to analyze the systemic and local concentration of cisplatin together with treatment-related toxicity after intrapleural HYALCIS application in an ovine model.
Methods
Animals, anesthesia and monitoring
The animal model used in this study was the sheep (Ovis aries), ovine species and Sardinian breeds. All animals were classified as ASA 1 or 2 on the basis of physical examination, electrocardiogram (ECG) and hematological parameters. Animals were purchased by a livestock farming located around Parma, authorized to this kind of study by the local AUSL (local health authority) in accordance with prevailing requirements. Animals were housed in appropriate experimental rooms at the Department of Veterinary Medicine of the University of Parma. The day before surgery, a chest radiogram was performed in order to exclude any infectious or inflammatory pulmonary diseases. A gynecological ultrasound was also carried out to look for possible ongoing pregnancy.
Food was withheld for 18 hours before anesthesia, but access to water was allowed at all times. A physical restraint was necessary to perform the premedication with diazepam (Diazepam 0.5%, Gellini, Aprilia, Italy) 0.5 mg/kg into the right cephalic vein through a 45 mm, 18-gauge venous cannula secured with a strap. The same catheter was used for drug and fluid administration throughout the entire surgical procedure. Flunixin meglumine 1.1 mg/kg (Finadyne®, Animal Health S.r.l., Milan, Italy) was administered intramuscularly (IM) to achieve perioperative analgesia and cefazolin 25 mg/kg intravenously (IV) (Cefazolina®, Merck, Frankfurter, Germany) was given to prevent postoperative infectious complications. Ringer’s lactate solution 10 mL/kg/h (Ringer lattato®, ACME, Cavriago, Reggio Emilia, Italy) was administered IV during surgery. Each sheep was pre-medicated with atropine 0.05 IM mg/kg (Atropina solfato®, ATI, Ozzano dell’Emilia, Bologna, Italy) followed by a bolus injection of diazepam 0.5 mg/kg IV (Valium®, Roche S.p.a., Segrate, Milan, Italy). Anesthesia was induced with propofol 3 mg/kg IV (Rapinovet®, Schering-Plough Animal Health, Harefield, UK). Immediately after the induction of anesthesia, a Magill endotracheal probe of 10 mm diameter was inserted into the trachea and the cuff was inflated. A stomach tube was inserted into the rumen. Anesthesia was maintained with 1.5–2% isoflurane (Isoflurane, Schering-Plough Animal Health, Harefield, Uxbridge, UK) in oxygen (2 L/min) via a semi-closed rebreathing system. Atracurium besylate 0.5 mg/kg IV (Tracrium®, GlaxoSmithKline, Stockley Park West, UK) was administered before the surgical incision. Intermittent positive pressure ventilation was used to maintain end tidal CO2 (EtCO2) in a range of 30–45 mmHg during surgery. The mechanical ventilator (ventilator Fabius, Dragër Italia, Corsico, Italy) was set with the following parameters: tidal volume 10 mL/kg, variable respiratory rate (RR) in order to ensure a minute volume of 150–200 mL/kg, ventilation pressure of 18–20 mmHg and positive end-expiration pressure of 5 mmHg. Intraoperative analgesia was achieved by intercostal nerve blocks between the 3rd and 7th intercostal space on the left side with lidocaine 2 mg/kg (Lidocaina 2%®, ATI, Ozzano dell’Emilia, Bologna, Italy) injected 10 minutes before the surgical incision and by an IV loading dose of 2 µg/kg fentanyl (Fentanest®, Pharmacia Italia, Nerviano, Italy) followed by a 5 µg/kg/h constant rate infusion during surgery. Postoperative analgesia was achieved by buprenorphine 10 µg/kg IM (Temgesic®, Schering-Plough Animal Health, Harefield, UK) administered 30 minutes before the end of surgery. Neostigmine 0.03 mg/kg IM (Prostigmina®, Meda Pharma, Milan, Italy) was given at the end of surgery. Cefazolin 25 mg/kg IM and flunixin meglumine 1.1 mg/kg IM were administered twice daily for 5 days after surgery.
Monitoring consisted of ECG, esophageal temperature (T°C), respiratory and heart rates (HR). We recorded EtCO2, partial saturation of hemoglobin (SpO2) and systolic (SAP), diastolic (DAP) and mean (MAP) arterial blood pressure by a non-invasive system. Cardiac and hemodynamic data and esophageal temperature were recorded by the monitor Guardian Byosis (Schiller, Esaote, Genova, Italy) while respiratory parameters were recorded by the monitor PM8050.
Drug and polymeric films
Polymeric films loaded with cisplatin (named HYALCIS form here on in) were used for this study. The physicochemical properties of these films together with their characteristics were extensively reported in previous papers (6,16). Briefly, cisplatin was purchased from Sigma-Aldrich (Saint Louis, USA). Hyaluronic acid (Ophthalmic HA, code 811240), the main component of the films, was kindly provided by Fidia Pharmaceuticals (Abano Terme, PD, Italy). Other reagents and excipients were purchased from A.C.E.F. (Fiorenzuola d’Arda, PC, Italy). The polymeric films were produced by lamination of the viscous solution containing cisplatin and all the excipients, followed by drying. For in vivo experiments, square films of 10×15 cm2 were cut and placed in a thermo-sealed sterile packaging until used. As we did in previous experiments (6,7) cisplatin was administered at a dosage of 100 mg/m2. Cisplatin-loaded films (HYALCIS) contained 1% w/w of drug on dry weight. Considering the weight of the animals (41.6±0.89 kg) and the cisplatin content in each film (1.12±0.06% w/w), all sheep received a dosage of cisplatin of 120 mg (mean dose of 119±6 mg). In order to cover the entire surface of the pleural cavity and to introduce 120 mg of cisplatin, 8 films with a surface area of approximately 150 cm2 were deposited on the pleural surface during the surgical procedure. Films were characterized for their thickness (0.82±0.03 mm) and their weight per square centimeter (10.7±0.6 mg/cm2) was calculated. Cisplatin concentration was determined by high-performance liquid chromatography (HPLC) (Pharm. Eur. 6.0). The same dosage of cisplatin (approximately 120 mg) was present in a 500 mL NaCl 0.9% w/v solution for intrapleural and intravenous administration (according to treatment groups; see Treatment groups section below).
Treatment groups
Twenty female Sardinian sheep, 2 years old, with a mean weight of 45.1 kg (range, 40–48 kg) were anesthetized to perform the surgical procedure. They were randomly assigned to the different treatment groups named: (I) control (500 mL NaCl 0.9% solution intrapleurally applied after the surgical procedure); (II) HYALCIS (hyaluronate films loaded with cisplatin intrapleurally applied after the surgical procedure); (III) cisplatin solution (500 mL NaCl 0.9% containing cisplatin) intrapleurally applied after the surgical procedure; (IV) cisplatin solution (500 mL NaCl 0.9% containing cisplatin) administered IV after the surgical procedure. The randomization was performed prior to surgery by means of random numbers sequence.
Surgical procedure and adjuvant therapy
Similarly to what we did in earlier experiments, and mimicking one of the two main surgical procedures, i.e., extra-pleural pneumonectomy, utilized in the surgical management of MPM in humans, a left pneumonectomy was performed in sheep. In this pharmacokinetic study, it was decided not to remove the parietal and diaphragmatic pleura, in order to evaluate the penetration and the absorption of cisplatin into and by these pleural layers.
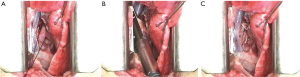
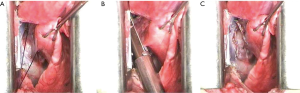
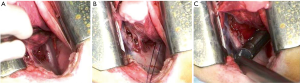
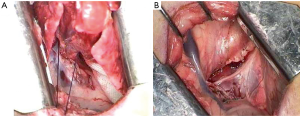
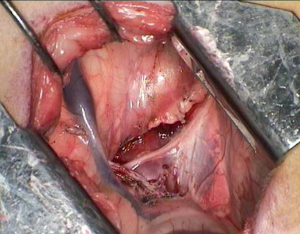
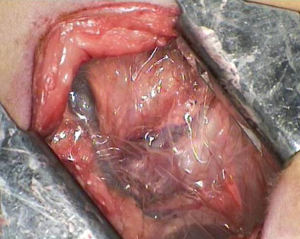
The anesthetized sheep was positioned in a right lateral decubitus. A left lateral thoracotomy at the 5th intercostal space was performed. A rib retractor was placed to facilitate all the procedures. Firstly, the superior pulmonary vein was isolated and sutured by mechanical vascular stapling (Figure S1). Secondly, the left main pulmonary artery was dissected and stapled (Figure S2). Then, the lower pulmonary vein was sutured by vascular mechanical stapler (Figure S3). Lastly, the left main bronchus was prepared and sutured by mean of mechanical stapling (Figure S4). After having removed the lung (Figure 1), the adjuvant therapy was administered according to previous randomization (see Treatment groups section). Animals belonging to group A, named control, received 500 mL NaCl 0.9% in the pleural cavity before closing the chest. Group B, named HYALCIS, was treated with intrapleural polymeric films. The films were removed from their packaging and were accurately placed into the cavity to cover the entire pleural surface in a sterile manner. The application of the device over the entire chest cavity was facilitated by their maneuverability. In all the cases, the films were well adherent to the mediastinal and parietal pleura at the end of the procedure (Figure 2). Sheep of group C had 500 mL NaCl with cisplatin intrapleurally applied before closing the chest. Animals belonging to group D received 500 mL NaCl with cisplatin by intravenous administration starting soon after lung removal and lasting 60 minutes. No pleural drainage was left in place. Chest cavity was closed with non-absorbable sutures. Animals were then extubated and strictly monitored for 4 hours before going back to their experimental rooms.
Cisplatin quantification in biological samples
Blinded cisplatin determination in the serum and tissue samples was performed by CM. All the samples were unfrozen just before starting the procedure. Quantification of cisplatin in biological specimens was obtained by determining elemental platinum using the ICP-MS X Series II (ThermoFisher Corporation, Waltham, MA, USA) equipped with an AS-500 autosampler (CETAC, Omaha, NE, USA).
The operating parameters of the ICP-MS instrument were as follows: RF power 1400 W; coolant gas flow 15.5 L∙min−1; auxiliary gas flow 0.98 L∙min−1; nebulizer gas flow 0.87 L∙min−1; nickel standard Xi cones; peak jumping data acquisition mode: dwell time 100 ms; duration time 60 s; standard resolution. The instrument optimization was performed daily with the auto-tune procedure to assure a response of at least 80,000 cps/µg∙L−1 for indium and 100,000 cps/µg∙L−1 for uranium in the high mass range. All the platinum isotopes were acquired to account for spectroscopic interferences, but only 195Pt was used for calculations. The instrument was calibrated over two detection ranges for concentrations between 0.01 and 1 ng/mL and from 1 to 10 ng/mL.
All the analyzed samples, i.e., homogenized tissue samples (800 mg) and blood serum (800 µL), were previously treated with aqua regia in a microwave assisted MLS-1200 MEGA apparatus (Milestone, Sorisole, Italy) equipped with an MDR-1000-6 rotor and diluted with ultrapure water to a final volume of 10 mL.
Blood samples and examination
A minimal physical restraint was necessary to perform the blood samples from the right cephalic vein (through a 45 mm, 18-gauge venous cannula secured with a strap, previously inserted and used during surgery). Blood samples were collected at the following intervals: 30 minutes, 1, 2, 4, 6, 24, 48, 72, 96, 168, 192 and 216 hours (day of the autopsy of the animals), starting from the time of drug administration/application. For one sheep treated with HYALCIS, monitoring and blood examinations continued until the 24th postoperative day. This decision came from the fact that animals were doing so well that the curiosity and interest to know the pharmacokinetic of the films over a long period of time were high. With this regard, an amendment to the experimental protocol was favorably assessed by the Local Veterinary Committee. A total of 6 mL of blood was collected in a plastic container. Two milliliters were used for standard blood test; 1 mL was centrifuged for 10 min at 1,000 ×g and the serum was tested for renal and hepatic parameters. Fifty microliters of serum were separated and stored at −80 °C until analysis of cisplatin determination; the remaining amount was also stored at −80 °C for possible future analysis. Hematological analysis was performed by electronic cell counter HEMA 5 (SEAC, Italy) using sterile tubes with the addition of lithium heparin as anticoagulant at a concentration of 35 UI heparin per milliliter blood (Sarstedt, Numbrecht, Germany). Electronic count of blood cells was determined setting the instrument for sheep. Biochemical clinical analysis (GOT, cod. 10745120; urea, cod. 11200666; and creatinine, cod. 10886674) were carried out by dry chemistry technique (Reflotron® System).
Sample preparation for histological examination
All animals except one were euthanized on the 9th postoperative day. As mentioned above, the last sheep was monitored until the 24th postoperative day and then euthanized. Afterwards, all of them were necropsied to evaluate gross and histological lesions induced by different experimental treatments. Several specimens from the surgical wound of the chest wall, bronchial stump, parietal pleura, diaphragm and pericardium have been collected. In order to histologically evaluate the possible treatment related toxicity, samples of liver, kidneys, hearth (left ventricular wall) and right lung were obtained. Additionally, samples of urine, parietal pleura, diaphragm and pericardium were collected for cisplatin content investigation. Samples of each tissue were immediately fixed in 10% neutral buffered formalin. After paraffin embedding, 5 µm thick sections were obtained with microtome (Leica), stained with hematoxylin and eosin (H&E) and Periodic acid-Schiff (PAS). Histological slides were examined with Nikon Eclipse E800 microscope (Nikon Corporation, Minato-Ku, Japan) using Nikon PLAN APO lenses. Sections were photographed at 4×, 10×, 20× and 40× (Nikon PLAN APO lenses) with Camera DIGITAL SIGHT DS-Fi1 (Nikon Corporation, Minato-Ku, Japan); pictures were acquired with DS Camera Control Unit DS-L2 (Nikon Corporation, Minato-Ku, Japan) and stored in USB device. Histological lesions were graded and classified based on the extension/distribution (focal, multifocal and diffuse) and severity (scant, mild, moderate, and severe).
Statistical analysis
Statistics were performed by using IBM SPSS Statistics ver. 24 (IBM, Amork, NY, USA) and P values <0.05 were considered to be significant. Data are expressed as mean and standard deviation. Analysis of variance (ANOVA) was performed to compare all the groups. Bonferroni correction was applied for comparison between all groups.
Results
Polymeric films
All polymeric films used in this study were flexible and resistant, providing a very good handling for intrapleural application during surgery. As in previous experiments, we observed that cisplatin loading had no significant effect on the mechanical properties of hyaluronate films. In all six animals, films were easily applied and homogeneously covered the parietal pleura and mediastinal structures.
Animal experiment
Twenty sheep were studied: 3 animals in group A (control), 6 animals in group B (HYALCIS), 6 animals in group C (intrapleural cisplatin solution) and 5 animals in group D (intravenous cisplatin solution). All the animals well tolerated the surgical operations and adjuvant treatments. Mean operation time was 113 minutes (range, 60–150 minutes). The animals were weighted preoperatively and the day of autopsy. Seventeen animals had a mean weight loss of 5.3 kg (range, 2–16 kg); 2 animals presented the same weight and 1 animal gained 5 kilograms. Two postoperative deaths occurred: one animal belonging to group B (on postoperative day 5) and one animal belonging to group C (on postoperative day 7). Both animals had a significant intraoperative blood loss and operation time was beyond 2 hours.
Pharmacokinetics
Plasmatic concentration of cisplatin
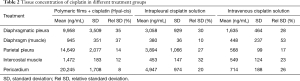
Pharmacokinetic parameters, including Cmax, Tmax, and the AUC at 9 days were calculated for animals receiving cisplatin as adjuvant therapy according to the abovementioned treatment groups (Table 1).
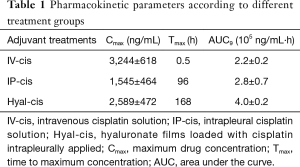
Full table
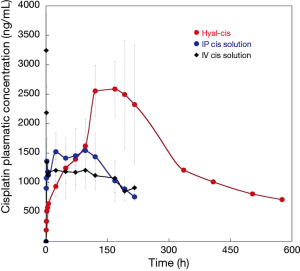
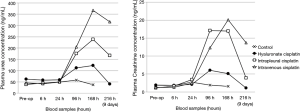
The plasmatic concentration of cisplatin during the first 24 hours after drug administration is presented in Figure 3. A plasmatic peak rapidly occurred after intravenous route of administration, and just as fast decreased. The drug concentration after intrapleural application of the cisplatin solution was higher than that achieved after HYALCIS application. In fact, as clearly shown (Figure 3), the release of the drug from the films was slow and progressive during the first hours.
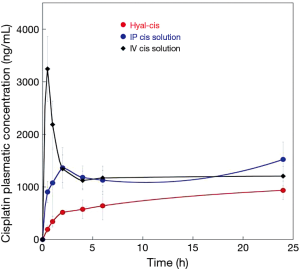
Figures 4 and 5 showed the trend and evolution of cisplatin concentrations during the entire study period in all treatments groups. The total blood concentration of cisplatin administered IV, subsequently to the drop that can be observed in the 6 hours from the treatment, continues to decrease slowly. After intrapleural treatment with cisplatin solution, the drug reached a plateau concentration starting from the 1st to the 5th postoperative day. The concentration achieved in the plateau phase, is not significantly different to that observed after the treatment with intravenous cisplatin solution (P=0.1). Regarding the intrapleural application of polymeric films loaded with cisplatin (HYALCIS), the drug concentration increased steadily and exceeded the one obtained after intravenous infusion in 2 days and that obtained after intrapleural administration in 5 days. Polymeric films led to a slow but constant increase of drug concentration up to the 7th day of therapy. Only 8 days after surgery, the decrease of plasmatic concentration of cisplatin has been observed. Starting from the 5th postoperative day and lasting until the 8th postoperative day (between 120 and 192 hours, postoperatively), animals receiving HYALCIS had a significantly higher cisplatin concentration than that registered in animals treated with intrapleural and intravenous cisplatin solution (Figure 4). More precisely, at 120h animals receiving HYALCIS had significant higher plasmatic concentration than animals treated with intrapleural (P=0.004) and intravenous (P=0.001) cisplatin solution. At 168, 192 and 216 hours from treatment administration, drug concentration remained significantly higher when animals received HYALCIS compared to animals which received intrapleural or intravenous cisplatin solution (P=0.001).
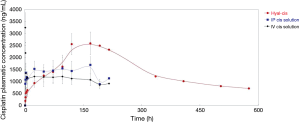
Because sheep treated with polymeric films loaded with cisplatin (HYALCIS) showed no sign of severe toxicity (see paragraph on treatment-related toxicity), it was decided to monitor one animal for a longer period of time. Thus, the profile of plasmatic cisplatin concentration was checked for an extended interval, i.e., up to 24 days after treatment administration (blood was collected at 14th, 17th, 21st and 24th days postoperatively). Figure 5 showed the concentrations of cisplatin during the 9 days following intravenous administration and intrapleural application of cisplatin solution, and during the 24 days following intrapleural treatment with polymeric films loaded with cisplatin. It is evident that the cisplatin concentration measured at 21 days is still close to 1 µg/mL and comparable to those obtained during the intravenous and intrapleural treatment with cisplatin solution after 9 days. This could be explained by the formation of a complex between hyaluronate and cisplatin that could be responsible for a reduction of the elimination rate of the drug (17).
Tissue concentration of cisplatin
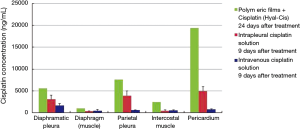
During animal autopsy (performed by Anna Maria Cantoni), biopsy specimens to measure tissue concentration of cisplatin were collected. In particular, samples were taken from diaphragmatic pleura, diaphragm (muscle), parietal pleura, intercostal muscle and pericardium (Table 2). Additional samples were taken for performing a histological examination aims to evaluate treatment-related toxicity.
Full table
Nine days postoperatively (time point of autopsy), it was observed that animals treated with hyaluronate films loaded with cisplatin (HYALCIS) had significantly higher tissue drug concentration compared to animals treated with intrapleural cisplatin solution and intravenous cisplatin solution (Figure 6). These significantly higher values were homogenously registered in all tissue samples (Table 2), including diaphragmatic pleura (P=0.001), diaphragm (muscle) (P=0.04), parietal pleura (P=0.001), intercostal muscle (P=0.03) and pericardium (P<0.001).
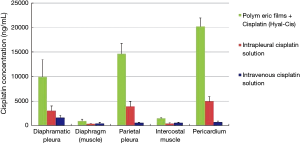
As abovementioned, we decided to monitor one sheep receiving intrapleural HYALCIS for 24 days. This was made to have more information regarding the pharmacokinetics of the polymeric films over a long period of time. The histogram represented in Figure 7 showed the tissue cisplatin concentration in the single HYALCIS animal observed for 24 days compared to all other animals treated with intrapleural and intravenous cisplatin solution euthanized on 9th postoperative day (same values shown in Figure 6). In all types of tissue samples, cisplatin concentrations of the animal monitored for 24 days were significantly higher than those registered nine days postoperatively in animals treated with intrapleural and cisplatin solution. These concentrations were extremely high and therefore highly significantly different for pericardium samples (P<0.001).
Treatment related toxicity
The experiment allowed also evaluating the renal, hepatic and cardiac toxicity associated with the treatments. Plasmatic level of urea and creatinine, selected as renal toxicity markers, were analyzed during the nine days of experiment and were reported in Figure 8. In both cases the plasmatic levels obtained for the group of animals treated with hyaluronate films loaded with cisplatin were lower than those obtained for the groups treated with intravenous and intrapleural cisplatin solutions for the duration of the study. In all stay groups, the peak of plasmatic concentration of urea and creatinine was reached at 168 hours (7th postoperative day) from treatment administration. In particular, at this time point, comparing animals receiving HYALCIS and animals receiving intravenous cisplatin solution, urea levels were significantly different (P=0.018). Conversely, urea did not differ significantly when comparing HYALCIS and intrapleural cisplatin solution (P=0.5), although its concentration was clearly lower (Figure 8). Looking at the plasmatic concentration of creatinine, animals treated with intrapleural HYALCIS had constantly lower levels than all other treatment groups, but the difference was not statistically significant (i.e., HYALCIS vs. intrapleural cisplatin solution: P=0.2; HYALCIS vs. intravenous cisplatin solution: P=0.1; HYALCIS vs. control: P=1.0).
In regards to hepatic toxicity, plasmatic levels of glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) were measured. The trend of the plasmatic concentration of these markers was not so clear as for renal markers. In fact, their fluctuations are difficult to interpret. Animals belonging to the control group (no adjuvant cisplatin treatment) also had an increase of hepatic markers. However, when we compared animals receiving HYALCIS with animals receiving intrapleural or intravenous cisplatin solution, GOT and GPT levels were not significantly different (P=0.1).
Histological examination of tissue samples
The results obtained from the measurements of different plasmatic toxicity markers, were confirmed by histological examination of tissue specimens collected during animal autopsies at the end of the experiment. With this regard, kidneys, liver and heart were evaluated.
Kidneys
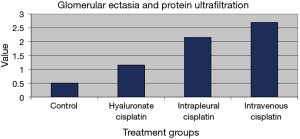
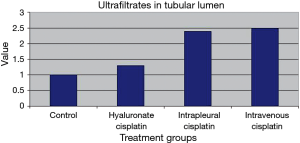
Several histopathological parameters of renal damage were evaluated, including glomerular ectasia and protein ultrafiltration and the presence of ultrafiltrates in the tubular lumen. They were studied giving a toxicity score from 0 to 3, depending on the severity of the lesions. Observations were carried out in single-blind conditions and included tissue samples from untreated sheep as control. Results are presented in Figures 9 and 10. Glomerular ectasia and protein ultrafiltration were less evident in animals treated with polymeric films loaded with cisplatin than in animals treated with intrapleural (P=0.3) and intravenous (P=0.029) cisplatin solution (Figure 9). Similarly, ultrafiltrates in the tubular lumen were significantly more present in sheep receiving intrapleural and intravenous cisplatin solution compared to sheep receiving polymeric films loaded with cisplatin (P=0.005 and P=0.004, respectively), thus confirming the slight toxicity of HYALCIS. With this regard, we found significant different scores comparing animals treated with intrapleural and intravenous cisplatin solution with controls (intrapleural cisplatin solution vs. control: P=0.009; intravenous cisplatin solution vs. control: P=0.007) (Figure 10). The scores observed after HYALCIS were not significantly different from those registered in control animals (P=1.0).
Liver
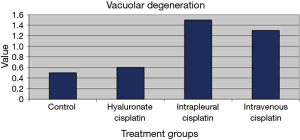
Similar results were obtained from histological examinations of hepatic tissue samples. Hepatic toxicity was related to vacuolar degeneration of hepatocytes or signs of steatosis. Figure 11 showed the toxicity scores assigned to the treatment groups. As far as vacuolar degeneration is concerned, we noticed significant lower scores given to HYALCIS groups compared to intrapleural and intravenous cisplatin solution (P=0.062 and P=0.044, respectively). In the same way, steatosis was less represented after HYALCIS treatment as compared with intracellular and intravenous cisplatin solution (data not shown).
Discussion
Since MPM is a discouraging and daunting tumor from both the prognostic and therapeutic point of view (10-12), targeted curative measures are becoming increasingly important more than ever (18-21). Loco-regional therapy is certainly one of these targeted measures (6,9,14). Having that in mind, some years ago we focused efforts on polymeric films loaded with cisplatin (HYALCIS) which we applied intrapleurally in an orthotopic rat model of MPM (7). In that study, the aim was to evaluate the efficacy of HYALCIS in reducing the volume of tumor recurrence after left pneumonectomy, assessing the adjuvant potency of the new formulation compared to intrapleural cisplatin solution. Firstly, we were able to find that animals treated with HYALCIS presented a significantly smaller tumor volume than animals treated with cisplatin solution. Secondly, the plasmatic and local concentrations of cisplatin measured at different time points, were significantly higher in animals receiving HYALCIS compared to animals belonging to other treatment groups. Despite this, the systemic toxicity observed in those animals treated with HYALCIS was significantly lower than all other treatment groups (7). We hypothesized that these encouraging data occurred as a result of the chemic-physical properties of the polymeric films, which allowed for a controlled release of the drug (7,16).
In the current study aiming to evaluate the pharmacokinetics and toxicity of the polymeric films loaded with cisplatin in a non-tumor-bearing ovine model, we randomly assigned to adjuvant treatment (after left pneumonectomy) 20 sheep. Interestingly, we observed significantly higher levels of cisplatin in plasma and pleural tissues after application of HYALCIS compared to intrapleural and intravenous cisplatin solution and at the same time with a significantly lower systemic toxicity. The plasmatic cisplatin concentrations were extremely high (up to 3,708 ng/mL) after HYALCIS treatment. The levels of cisplatin registered in pathological tissue samples were up to more than 6 times as high as those observed in plasma sample, and specifically they reached around 10,000 ng/mL in diaphragmatic pleura, about 15,000 ng/mL in parietal pleura and 20,000 ng/mL in the pericardium. These data prompted us to hypothesize that the therapeutic potential of polymeric films loaded with cisplatin could be very high. This could mean having the opportunity to expose tumor cells to high concentrations of chemotherapeutic drug, which is crucial to the strengthening of the antitumor activity.
We believe these findings are very important, mostly because loco-regional treatments are a very promising option for the treatment of MPM. In fact, other systemic or intrapleural alternatives have been under investigation by different working groups (22-26). Immunotherapy, in the form of immune checkpoint blockade, immunotoxin therapy, anticancer vaccines, oncolytic viral therapy, and adoptive cell therapy are the most common methods of immunotherapy currently being used in the clinical setting (22). Oncolytic viruses, which work by infecting and lysing tumor cells, are an exciting field of oncological research. Vaccinia, an enveloped virus that is largely immunogenic and at the same time safe when given to humans, has been shown to be able to kill mesothelioma cell lines in vitro and also in an orthotopic mouse model of MPM. Moreover, tumor volume was significantly decreased (27).
These encouraging data led to a clinical phase I study where the GL-ONC1 vaccinia virus was intrapleurally applied to patients with malignant pleural effusions. Data are not yet available (22). Attenuated measles virus (MV) is comparable to vaccinia; at the moment, there is a recruiting phase I clinical study aiming to examining the intrapleural delivery of MV therapy for patients with stage I–IV or recurrent MPM (22).
Immunotherapy can also be associated with radiotherapy [including intensity modulated radiation therapy (IMRT)]and chemotherapy (23,24). The potential benefit of short course high dose radiation on the immune system has been reported, and that could be the rationale for the combination of the two modality treatments (23). On the other hand, some chemotherapeutic drugs have been shown to induce cell death and therefore can stimulate an immune response against tumor cells (23-25). Some years ago, we assessed the effect of intrapleural immuno-chemotherapy on the extent of local tumor recurrence in an established rat model of MPM (6). Synthetic TLR9 ligands containing un-methylated cytosine phosphate guanosine oligodeoxynucleotide (CpG-ODN) were used. We found that CpG reduces the volume of tumor recurrence after surgical resection when applied intrapleurally either alone or in combination with chemotherapy, in the form of cisplatin loaded to the fibrin sealant Vivostat® (6). In addition, immuno-chemotherapy resulted in an increased recruitment of inflammatory cells to the site of tumorigenesis and elicited higher level of tumor growth inhibiting cytokines.
As mentioned above, intrapleural approaches present the advantage to minimize the adverse effects of chemotherapy, while maximizing its therapeutic efficacy by obtaining high concentrations of the chemotherapeutic drug within the tumor tissue (28). Pemetrexed is currently being used, in combination with cisplatin, as a first-line treatment in patients with MPM. However, when used alone, pemetrexed does not have relevant anti-tumor activity in vivo. This could due to either inadequate delivery of sufficient concentrations to tumor tissue or dose-limiting side effects. Essam Eldin et al., in order to overcome these problems and try to enhance specific anti-tumor activity, encapsulated pemetrexed into a liposomal delivery system (28). They studied different formulations of liposomal pemetrexed and tested their efficacy in vitro on several mesothelioma cell lines. They found that, when encapsulated within fluid liposomes, pemetrexed showed a superior in vitro cytotoxic effect against mesothelioma tumor cells, compared to pemetrexed encapsulated within solid liposomes. They concluded that pemetrexed encapsulated within fluid liposomes might exert a potent therapeutic efficacy, superior to that observed with free pemetrexed (28). In our previous experiment, we were also interested in assessing the potential effect of pemetrexed loaded onto our polymeric films (alone and in association with cisplatin) in the rat mesothelioma model (data not published). We were able to find a further reduction of tumor recurrence in animals receiving polymeric films loaded with cisplatin and pemetrexed compared to animals receiving polymeric films loaded with cisplatin alone, but the difference was not statistically significant (data not shown). Considering that animals treated with the combined therapy had a slightly higher (although not significant) hepatic toxicity, we decided to continue our experimental study utilizing only polymeric films loaded with cisplatin, at least for the time being.
In summary the film, as a consequence of its mechanical properties, can be easily adapted to the site of application, with the opportunity (as in this case) to cover the entire pleural surface homogeneously. In any case the delivery system is versatile and allows bending, folding, cutting (if necessary) and any deformation needed to ease its application in a surgical setting. It provides a prolonged release of cisplatin, maintaining constant the anticancer activity and seemingly reducing adverse effects.
In addition, it is possible to imagine that by mean of this technology, different film formulations could be manufactured with the aim to further control the drug release, particularly in an attempt to prolong as much as possible the presence of the drug in the pleural cavity. Moreover, the manufacturing technology of the film allows the construction of mono- or multilayer films employing different polymers or presenting different drug loading. The aim would be to modulate the drug release according to the administration plan.
For example, considering films with a single layer structure (as those used in our experiments so far), cisplatin could be released by both surfaces of the film, that means towards the chest wall tissue to which it has been applied and towards the free volume of the pleural cavity. But thinking about a double layer film, in which one external layer would not be loaded with the drug, it might be possible to direct the drug release towards the pleural surface with the effect of further concentrate and therefore increase the drug concentration in the target tissue.
Conclusions
In conclusion, polymeric films loaded with cisplatin were found to assure significantly higher and more prolonged plasmatic and loco-regional drug concentrations than intrapleural and intravenous cisplatin solution, without increasing the toxicity in a non-bearing-tumor ovine model of MPM.
The following step will be a phase I clinical study in MPM patients not responding to other therapies, with the following specific aims: (I) to determine the dose-limiting toxicities (DLTs) of polymeric films loaded with cisplatin for the intrapleural treatment of malignant pleural tumors and to identify the recommended phase II dose (RP2D); (II) to characterize the pharmacokinetics of intrapleural polymeric films loaded with cisplatin; (III) to determine the time to pleural effusion recurrence as a sign of antitumor activity.
In this regard, on 29 August 2016, orphan designation (EU/3/16/1719) was granted by the European Commission to PlumeStars s.r.l., Italy, for polymeric films loaded with cisplatin (HYALCIS) for the treatment of malignant mesothelioma (29). More recently, on 02 May 2017, our orphan-drug designation request of hyaluronate films loaded with cisplatin (HYALCIS) implant was granted for treatment of mesothelioma by the Office of Orphan Product Development of the Food and Drug Administration (FDA, Silver Spring, MD, USA).
Acknowledgements
We would like to thank Dr. Francesco Oricchio and Dr. Francesca Pellegrino for helping during the surgical procedures. We are indebted to all the veterinary staff for technical assistance during the experiment. We also would like to remember Stefano Zanichelli, full professor of veterinary surgery who passed away on 9th December 2015. We really wish to thank him for, over the past years, working tirelessly to support scientific research and innovative treatments. This work was supported by CHIESI Farmaceutici S.p.A. (Parma); Transfer Oil S.p.A. (Colorno, PR); Famiglia Gigetto Furlotti (Parma); Associazione Volontaria Promozione Ricerca Tumori (A.VO.PRO.RI.T.) (Parma); Associazione Augusto per la Vita (Novellara, Reggio Emilia); Circolo Aquila Longhi (Parma) and Fondazione Monte di Parma (Parma).
Footnote
Conflicts of Interest: P Colombo is the founder and CEO of the start-up Plumestars s.r.l.; F Sonvico has received support for the development of polysaccharide films from Plumestars s.r.l. The other authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Local Veterinary Committee (Prot. N 92/11) and the experiments were conducted in accordance with the Guiding Principles in the Care and Use of animals (Legislative Decree 116/92) and Regional Decree 20/2002 of Emilia Romagna Region, Italy.
References
- Novello S, Pinto C, Torri V, et al. The Third Italian Consensus Conference for Malignant Pleural Mesothelioma: State of the art and recommendations. Crit Rev Oncol Hematol 2016;104:9-20. [Crossref] [PubMed]
- Świątkowska B, Szeszenia-Dąbrowska N. Mesothelioma continues to increase even 40 years after exposure - Evidence from long-term epidemiological observation. Lung Cancer 2017;108:121-5. [Crossref] [PubMed]
- Ogunseitan OA. The asbestos paradox: global gaps in the translational science of disease prevention. Bull World Health Organ 2015;93:359-60. [Crossref] [PubMed]
- van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 2013;5:E254-307. [PubMed]
- van Zandwijk N. Clinical practice guidelines for malignant pleural mesothelioma. J Thorac Dis 2013;5:724-5. [PubMed]
- Ampollini L, Soltermann A, Felley-Bosco E, et al. Immuno-chemotherapy reduces recurrence of malignant pleural mesothelioma: an experimental setting. Eur J Cardiothorac Surg 2009;35:457-62. [Crossref] [PubMed]
- Ampollini L, Sonvico F, Barocelli E, et al. Intrapleural polymeric films containing cisplatin for malignant pleural mesothelioma in a rat tumour model: a preliminary study. Eur J Cardiothorac Surg 2010;37:557-65. [Crossref] [PubMed]
- Marulli G, Breda C, Fontana P, et al. Pleurectomy-decortication in malignant pleural mesothelioma: are different surgical techniques associated with different outcomes? Results from a multicentre study. Eur J Cardiothorac Surg 2017;52:63-9. [Crossref] [PubMed]
- Opitz I, Erne BV, Demirbas S, et al. Optimized intrapleural cisplatin chemotherapy with a fibrin carrier after extrapleural pneumonectomy: a preclinical study. J Thorac Cardiovasc Surg 2011;141:65-71. [Crossref] [PubMed]
- Corradi M, Goldoni M, Alinovi R, et al. YKL-40 and mesothelin in the blood of patients with malignant mesothelioma, lung cancer and asbestosis. Anticancer Res 2013;33:5517-24. [PubMed]
- Bonelli MA, Digiacomo G, Fumarola C, et al. Combined Inhibition of CDK4/6 and PI3K/AKT/mTOR Pathways Induces a Synergistic Anti-Tumor Effect in Malignant Pleural Mesothelioma Cells. Neoplasia 2017;19:637-48. [Crossref] [PubMed]
- Mozzoni P, Ampollini L, Goldoni M, et al. MicroRNA Expression in Malignant Pleural Mesothelioma and Asbestosis: A Pilot Study. Dis Markers 2017;2017:9645940.
- Aigner KR, Selak E, Gailhofer S. Isolated thoracic perfusion with chemofiltration for progressive malignant pleural mesothelioma. Onco Targets Ther 2017;10:3049-57. [Crossref] [PubMed]
- Opitz I, Lardinois D, Arni S, et al. Local recurrence model of malignant pleural mesothelioma for investigation of intrapleural treatment. Eur J Cardiothorac Surg 2007;31:773-8. [Crossref] [PubMed]
- Guerrini VH, Filippich LJ, Cao GR, et al. Pharmacokinetics of cefaronide, ceftriaxone and cefoperazone in sheep. J Vet Pharmacol Ther 1985;8:120-7. [Crossref] [PubMed]
- Sonvico F, Barbieri S, Colombo P, et al. Physicochemical and pharmacokinetic properties of polymeric films loaded with cisplatin for the treatment of malignant pleural mesothelioma. J Thorac Dis 2017. [Epub ahead of print].
- Cai S, Xie Y, Davies NM, et al. Pharmacokinetics and disposition of a localized lymphatic polymeric hyaluronan conjugate of cisplatin in rodents. J Pharm Sci 2010;99:2664-71. [Crossref] [PubMed]
- Porpodis K, Zarogoulidis P, Boutsikou E, et al. Malignant pleural mesothelioma: current and future perspectives. J Thorac Dis 2013;5 Suppl 4:S397-406. [PubMed]
- Opitz I. Management of malignant pleural mesothelioma-The European experience. J Thorac Dis 2014;6 Suppl 2:S238-52. [PubMed]
- Wald O, Sugarbaker DJ. Malignant pleural mesothelioma: key determinants in tailoring the right treatment for the right patient. J Thorac Dis 2017;9:485-9. [Crossref] [PubMed]
- Takuwa T, Hasegawa S. Pleurectomy/decortication for malignant pleural mesothelioma. J Thorac Dis 2017;9:460-1. [Crossref] [PubMed]
- Dozier J, Zheng H, Adusumilli PS. Immunotherapy for malignant pleural mesothelioma: current status and future directions. Transl Lung Cancer Res 2017;6:315-24. [Crossref] [PubMed]
- Wu L, de Perrot M. Radio-immunotherapy and chemo-immunotherapy as a novel treatment paradigm in malignant pleural mesothelioma. Transl Lung Cancer Res 2017;6:325-34. [Crossref] [PubMed]
- Chan WH, Sugarbaker DJ, Burt BM. Intraoperative adjuncts for malignant pleural mesothelioma. Transl Lung Cancer Res 2017;6:285-94. [Crossref] [PubMed]
- Okabe K. Intraoperative intracavitary hyperthermic chemotherapy for malignant pleural mesothelioma. Ann Transl Med 2017;5:233. [Crossref] [PubMed]
- Facchetti G, Petrella F, Spaggiari L, et al. Malignant Pleural Mesothelioma: State of the art and advanced cell therapy. Eur J Med Chem 2017.266-70. [Crossref] [PubMed]
- Belin LJ, Ady JW, Lewis C, et al. An oncolytic vaccinia virus expressing the human sodium iodine symporter prolongs survival and facilitates SPECT/CT imaging in an orthotopic model of malignant pleural mesothelioma. Surgery 2013;154:486-95. [Crossref] [PubMed]
- Essam Eldin N, Elnahas HM, Mahdy MA, et al. Liposomal pemetrexed: formulation, characterization and in vitro cytotoxicity studies for effective management of malignant pleural mesothelioma. Biol Pharm Bull 2015;38:461-9. [Crossref] [PubMed]
- Public summary of opinion on orphan designation: cisplatin for the treatment of malignant mesothelioma. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/orphans/2016/10/human_orphan_001829.jsp&mid


