Imaging of malignant pleural mesothelioma: it is possible a screening or early diagnosis program?—a systematic review about the use of screening programs in a population of asbestos exposed workers
Introduction
Malignant pleural mesothelioma (MPM) is an uncommon neoplasia.
In 2016, mesothelioma of any site had an incidence rate of 2.0 cases per 100,000 men and 0.3 per 100,000 women in Italy (1), with pleural localization as the commonest site (93% of cases) (2). However, in some geographic areas, incidence has considerably increased over the last decades due to professional exposure to asbestos, whose correlation with the onset of mesothelioma is now clearly established. Because of professional exposure MPM affects male much more then female, and the latency period let the tumour develop and manifest about 30 years after exposure.
Pleural mesothelioma is notoriously a tumour with high mortality rate and has a mean survival of 10 months (AIOM Guidelines, 2016) (3), so that cause-specific mortality well reflects the incidence of disease.
Clinical manifestations of mesothelioma are not specific, so the diagnosis of malignant mesothelioma can be difficult with symptoms and clinical findings that can mimic and be mimicked by other diseases.
Among available imaging tools chest X-ray is the first-line approach (4) and it is generally abnormal in advanced stages of disease, showing in most cases the presence of unilateral pleural effusion or pleural thickening. Diagnostic accuracy of chest X-ray is obviously low but can be adequate in early stages to suspect the disease. Suspicion is based on the presence of some radiographic signs easily observed: drug-resistant unilateral pleural effusion, unilateral lobulated pleural thickening with or without thickening pleural fissures, multiple masses with peripheral distribution, and loss of volume in the hemithorax involved. Above all unilateral pleural effusion was found in 30% to 80% of the early-stage disease and it often hide the underlying neoplasia (5,6). It must therefore be considered suspicious, especially in patients with known or probable exposure.
Contrast-enhanced volumetric computed tomography (CT) scan of the chest represents the gold standard to the imaging diagnosis. The features of malignant pleuropathy have been well described in literature and can be summarized as: circumferential pleural thickening (pleural rind); thickened mediastinal pleura; nodular or lobular borders within the internal profile; irregular borders of the external profile (infiltration of the wall); mediastinal and pericardial infiltration, lymph nodes in extra pleural fat tissues (7-12). Pleural effusion is an unspecific sign and pleural calcific or not calcific plaques are indicative of asbestos exposure but not of neoplastic pathology (8).
Main differential diagnosis is metastatic pleuropathy, which have a more “discrete” and discontinuous distribution, while mesothelioma tends to manifest with a continuous distribution, like a rind. However, there are not specific diagnostic criteria to perform differential diagnosis between metastatic pleuropathy and MPM; considering that pleural metastasis is more frequent than MPM with a ratio of above 95:5.
MPM’s CT staging is similarly based on some characteristic features: thickening of the visceral pleura, which is discernible only when effusion is present, and infiltration of contiguous structures such as lung parenchyma, mediastinal organs, chest wall, diaphragm and peritoneum, more visible with multiplanar reconstruction. CT underestimates some features of T staging such as chest wall and peritoneum invasion; furthermore, it has low diagnostic accuracy for N staging (about 50%). Nevertheless, CT is considered mandatory for patients’ staging and follow-up (13-15).
Magnetic resonance imaging (MRI) is not routinely performed in the clinical practice for patients with MPM; there is some evidence in literature that MRI signal could be useful to differentiate malignant pleuropathy from benign pleural fibrous plaque (12,16). MRI is better than CT for detecting invasion of chest wall, mediastinal and nervous structures as brachial plexus, and peritoneum (15) and is generally reserved for those patients eligible for surgical treatment.
18F-FDG-PET/CT can show metabolic activity at the level of pleural thickening in case of mesothelioma, allowing differential diagnosis with fibrous pathology in most cases. It can demonstrate lymphadenopathy and distant metastasis with sensibility of 90% and specificity close to 80%. PET/CT is better than CT and MRI for N and M staging, improving inter-observer agreement and preoperative staging accuracy (15,17,18). In case of metastatic pleuropathy, PET/CT could detect a primitive tumour.
Patients with remarkable pleural thickening without effusion can perform trans-parietal ultrasound (US)- or CT-guided biopsy. The sensibility of trans-parietal biopsy is between 50% and 85% (19), lower than thoracoscopy; for this reason, it is therefore not routinely recommended for definitive diagnosis of mesothelioma, except in patients non-suitable to thoracoscopy. Indeed, when MPM is suspected by clinical or radiological data the diagnostic accuracy of thoracoscopy is very high, exceeding 90%, and complications occur in less than 10% of cases (14,15,20).
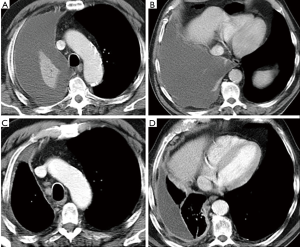
During the follow-up, patients are followed with clinical controls and evaluated within the multidisciplinary group whenever a recurrence of the disease is suspected. CT is performed every 3–4 months during the first year of follow-up (Figure 1) or anytime when a recurrence is suspected. Then, radiological follow-up is defined for each patient on clinical basis.
Despite available treatments MPM has a poor short- and medium-term prognosis with main symptoms characterized by pain and dyspnoea, due to pleural effusion and infiltration of chest wall.
Finally, as regard the possibility of early diagnosis, it is necessary to consider that MPM is a fast-growing tumour with high mortality rate and overall survival less than 1 year (1). For this reason, even if the main risk factor is well known (asbestos exposure) there is no screening protocol suitable to perform an early diagnosis and to reduce the mortality. By the way, screening protocols are available for the other recurrent pathologies in exposed subjects, such as lung cancer.
CT screening in asbestos exposed subjects
Low-dose CT (LDCT) screening of lung
High resolution CT (HRCT) for asbestos-related interstitial lung disease (asbestosis) requires sequential scanning technique with full dose (kV 100–140, mAs 200–250), and slice thickness of 1 mm (21); the axial sections are acquired with 10-mm intervals at different level of the chest with patient in prone position and at full inspiration. This technique allows the use of a low-dose (around 2–3 mSv). Main limitation of this technique is however the gap between noncontinuous slices that causes problems in the detection of small lung nodules (<1 cm).
For this reason, a volumetric acquisition without interspace of entire lungs is necessary, with the disadvantage of a higher-dose exposure (5–7 mSv). From the 1990s, a LDCT volumetric acquisition technique was developed; it is based on milliampere reduction (mAs 20–40) and continuous sections of 1 or 1.5 mm acquired with a dose exposure below 2 mSv. This technique allows a high spatial resolution useful for lung parenchymal examination and pulmonary nodules identification.
Many observational and randomized studies about the use of LDCT in lung screening protocols were performed. For instance, a large-scale observational study, the American ELCAP, was conducted from the late 1990s, demonstrating that LDCT has a greater sensibility than chest X-ray to detect tumour at early and treatable stage in screening population (22,23).
More recently, the National Lung Screening Trial (NLST) demonstrated a cause-specific mortality reduction in strong smokers submitted to lung cancer LDCT screening. This is an US randomized controlled multicentre trial (24) with around 50,000 subjects enrolled, randomized in an active group (annual LDCT screening test) and control group (chest X-ray). The trial demonstrated a significant reduction of mortality in LDCT screening group such as 20% from lung cancer and 7% from all causes, so the study was suspended after 2 years because of excess benefit. Nowadays, US Medicare covers the costs of lung cancer screening in strong smokers (25). In addition, an Italian randomized trial (ITALUNG) recently published the definitive data (26), showing a decrease of overall mortality rate (17%) and of lung cancer mortality rate (30%) in the group annually screened by LDCT.
The above-mentioned studies confirmed the role of LDCT as a screening tool for detecting lung cancer in subjects at risk, although European researchers are still discussing the possible role and cost/benefit ratio of screening programs in large cohorts.
HRCT and LDCT in Helsinki Declaration
Based on this evidence, screening for asbestos-related disease with LDCT was considered in the literature. In the recent publication of Helsinki criteria about asbestos, asbestosis and cancer, the possibly role of lung cancer screening was evaluated and recommended (27) in subjects exposed to asbestos.
In the first part, the document (Area 1) suggests the role of lung cancer screening with LDCT in exposed subjects and recommends to use a standardized method and to create a large database of the results.
Inclusion criteria for screening in exposed subjects are defined:
- Workers with any history of asbestos exposure and with smoking history equivalent to subjects enrolled in NLST;
- Workers exposed with or without a smoking history, but with overall risk equivalent to those enrolled in the NSLT.
Finally, the paper suggests to concentrate the screening activity in a single regional or national unit, in order to optimize procedures and standardize results analysis.
The second part of the Helsinky declaration (Area 2) summarizes the role of CT in surveillance/screening of asbestos-exposed workers. Medical legal issues justify the use of CT more than a scientific evidence of benefit for exposed subjects with many differences between nations and medical centres. There are some cases where CT may be useful in diagnosis of asbestos-related diseases: borderline chest X-ray (ILO 0/1 or 1/0), discrepancy between chest X-ray and pulmonary function tests and extensive pleuropathy that hides lungs. The Helsinki protocol recommends the use of an international classification [ICOERD criteria (28)] for pulmonary and pleural abnormalities detected on CT to identify both malignant and non-malignant asbestos diseases. The ICOERD system, comparable to the 1980 ILO international classification of radiographs for pneumoconiosis, defines the criteria for diagnosis of asbestosis using standard reference images. In particular, bilateral basal irregular opacities (sum grade ≥2–3) or bilateral honeycombing (sum grade ≥2) would be sufficient to represent fibrosis and to diagnose asbestosis.
LDCT lung cancer screening in asbestos-exposed workers
From 2002 to 2009, the results of some lung cancer screening trials with LDCT on asbestos-exposed workers were published (29-34). Unfortunately, in these studies CT image acquisition and reconstruction algorithms are different as well as technical parameters (mA, slice thickness, reconstruction interval, additional HRCT slices, etc.); furthermore, the criteria to determine a positive test are not homogeneous. Most of all use ELCAP, I-ELCAP criteria (22,23) or a modification of these (29,31,32,35) to determine a positive test for non-calcific nodule; in other trials the presence of pleural abnormalities is a criterion considered for a positive test (29-32), but the definitions of pleural involvement and subsequent work-up are various and not always included in the protocol study.
These differences make it difficult to compare the results of the trials, although a recent meta-analysis (36) showed that lung cancer prevalence in asbestos-exposed workers screening is equivalent to that reported in strong smokers.
For instance, the observational study of Fasola et al. (33) performed in Udine on 1,045 asbestos-exposed workers (mean age, 58 years; mean exposure time, 30 years; 66% smokers) identified 9 lung cancers (detection rate 1%), 8 at stage 1; no mesothelioma at baseline was found. The authors concluded that lung screening with LDCT is useful in asbestos-exposed subjects at least as in strong smokers. On the contrary, Mastrangelo et al. (32), in an observational study performed in Padova, evaluated over 1,119 exposed subjects (mean age, 57 years; mean exposure, 123 fibers/mL × year; 65% smokers) and identified 5 lung cancers (detection rate 0.4%), 1 at stage 1; no mesothelioma was found. In this paper lung cancer screening with LDCT was not considered cost-effective.
Even in lung cancers screening designed for strong smokers enrolled with various criteria, were occasionally included asbestos-exposed subjects (22-24,37). In I-ELCAP study, subjects with professional asbestos exposure enrolled were about 5% (23), while in NLST (38) study 4.6% of active group and 4.8% of control group were asbestos exposed workers. Data resulting from these subgroups of subjects could be interesting, because they compare smokers or ex-smoker with a similar risk profile.
It should be also mentioned a paper published by Remy-Jardin et al. (39) that compared the interstitial abnormalities evaluated on asbestos-exposed subjects both with prone HRCT and supine LDCT. The authors demonstrated that LDCT is an accurate method for diagnosis of asbestos-related pathology because it allows the diagnosis of lung interstitial abnormalities with an accuracy comparable to HRCT allowing the detection of pleural lesions and parenchymal nodules. Furthermore, no differences were found to classify parenchymal abnormalities in both scans; it is due to full inspiration during short-time examination in supine position, which prevents the development of areas of increased density in lung dependent, that could mask or mimic signs of interstitial pathology. A negative LDCT is therefore considered sufficient to exclude pleural and pulmonary asbestos-related disease.
Discussion and conclusions
Lung cancer is a very important problem in asbestos-exposed workers, with an incidence and mortality higher than MPM. In our clinical data (unpublished), 21 lung tumours (1.38%) and only 2 MPM (0.13%) among 1,513 patients were detected.
In the literature, Ohar et al. (40) reported a 1.9% lung cancer prevalence among 3,383 asbestos-exposed workers with a low ILO score. Ameille et al. (41) in the French National Monitoring Program reported an estimated annual incidence of lung cancer in asbestos-exposed workers between 2,086 and 4,172 (1990) and an annual MPM incidence between 646 and 800 [1998–2003]. Moreover, it is known that the interaction between asbestos exposure and smoking habit has an additive or multiplicative effect for developing lung cancer (42).
Even more impressive are the results of a meta-analysis of McCormack et al. (43), based on 55 different cohorts, which reported a lung cancer mortality at least twice as that from MPMs on asbestos exposed subjects, with the only exception of crocidolite. In a more specific way, a meta-analysis of Ollier et al. (36) evaluated seven lung screening studies in asbestos-exposed workers, and reported 49 lung tumours between 5,074 asymptomatic subjects, with a prevalence of 1.1%; 18 tumours (36.7%) were at stage I and underwent radical surgery. Authors conclusion was that LDCT screening in asbestos-exposed subjects could reduce mortality as observed in strong smokers, and should not be denied, especially in patients with both exposures.
From a methodological point of view, an ideal integrating screening test for asbestos-related pathologies would be a CT technique, that provides information on parenchymal nodules, interstitial abnormalities, as well as pleural pathology. R Jardin underlies as LDCT is an accurate method for diagnosis of asbestos-related interstitial pathology, with accuracy similar to HRCT and simultaneous allows evaluation of pleural lesions and lung parenchymal nodules, thus providing the best technique for a possible screening among asbestos-exposed people (39).
A distinctive feature of screening programs with LDCT in exposed subjects compared to surveillance protocols is that the protocol requires subsequent controls at constant and predetermined intervals, usually 1 year, even with a baseline negative test. The purpose of the annual interval (44) is to allow the detection of any changes between successive examinations, such as the occurence of a new nodule or the growth of some pre-existing nodules, which are elements of suspicion.
Intervals between CT scans are determined on the basis of time of growth of the most common lung cancers found in risk population, generally a peripheral form with histology of adenocarcinoma (45,46). Unfortunately, MPM, as well as small cell lung cancer (SCLC), is a tumour with a very rapid growth rate that eludes the screening intervals. Therefore, these tumour histotypes (MPM and SCLC) are not the object for screening programs, although they are occasionally identified and usually reported as interval cancer (Figure 1).
In conclusion could be useful to emphasize the recommendation of Helsinki Criteria, which says that “it is reasonable to recommend that adults with asbestos exposure be evaluated for eligibility for lung cancer screening. Those adults with prior exposure to asbestos who are in reasonably good and who are at or above the risk threshold set for participation in the NLST, whether based on smoking history, the combination of asbestos exposure and smoking history, or asbestos exposure alone, should be considered for screening for lung cancer” (27).
Although the research is very active in this field, the current point of view is that MPM screening is not feasible; instead lung cancer screening in asbestos-exposed subjects, with a careful evaluation of risk factors, is not only possible, but also desirable.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- I Numeri del Cancro in Italia. 2016. Available online: http://www.registri-tumori.it/PDF/AIOM2016/I_numeri_del_cancro_2016.pdf
- V Rapporto. Il Registro Nazionale Mesoteliomi. INAIL, Roma 2015. Available online: https://www.inail.it/cs/internet/docs/ucm_207055.pdf
- Linee guida. Mesotelioma pleurico. AIOM 2016. Available online: http://www.aiom.it/professionisti/documenti%2Dscientifici/linee%2dguida/mesotelioma%2dpleurico/1,2811,1,
- Miller A, Widman SA, Miller JA, et al. Comparison of x-ray films and low-dose computed tomographic scans: demonstration of asbestos-related changes in 2760 nuclear weapons workers screened for lung cancer. J Occup Environ Med 2013;55:741-5. [Crossref] [PubMed]
- Alexander E, Clark RA, Colley DP, et al. CT of malignant pleural mesothelioma. AJR Am J Roentgenol 1981;137:287-91. [Crossref] [PubMed]
- Miller BH, Rosado-de-Christenson ML, Mason AC, et al. From the archives of the AFIP. Malignant pleural mesothelioma: radiologic-pathologic correlation. Radiographics 1996;16:613-44. [Crossref] [PubMed]
- Leung AN, Müller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol 1990;154:487-92. [Crossref] [PubMed]
- Falaschi F, Boraschi P, Musante F, et al. The computed tomographic diagnosis of malignant pleural mesothelioma. A multicenter study. Radiol Med 1992;84:43-7. [PubMed]
- Metintas M, Ucgun I, Elbek O, et al. Computed tomography features in malignant pleural mesothelioma and other commonly seen pleural diseases. Eur J Radiol 2002;41:1-9. [Crossref] [PubMed]
- Wang ZJ, Reddy GP, Gotway MB, et al. Malignant pleural mesothelioma: evaluation with CT, MR imaging, and PET. Radiographics 2004;24:105-19. [Crossref] [PubMed]
- Zhou H, Tamura T, Kusaka Y, et al. Evaluation of the efficacy of the guideline on reading CT images of malignant pleural mesothelioma with reference CT films for improving the proficiency of radiologists. Eur J Radiol 2013;82:169-76. [Crossref] [PubMed]
- Coolen J, De Keyzer F, Nafteux P, et al. Malignant pleural mesothelioma: visual assessment by using pleural pointillism at diffusion-weighted MR imaging. Radiology 2015;274:576-84. [Crossref] [PubMed]
- Flores RM. The role of PET in the surgical management of malignant pleural mesothelioma. Lung Cancer 2005;49 Suppl 1:S27-32. [Crossref] [PubMed]
- Rice DC, Erasmus JJ, Stevens CW, et al. Extended surgical staging for potentially resectable malignant pleural mesothelioma. Ann Thorac Surg 2005;80:1988-92. [Crossref] [PubMed]
- Pinto C, Novello S, Torri V, et al. Second Italian Consensus Conference on Malignant Pleural Mesothelioma: State of the art and recommendations. Cancer Treat Rev 2013;39:328-39. [Crossref] [PubMed]
- Falaschi F, Battolla L, Mascalchi M, et al. Usefulness of MR signal intensity in distinguishing benign from malignant pleural disease. AJR Am J Roentgenol 1996;166:963-8. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Porcel JM, Hernández P, Martínez-Alonso M, et al. Accuracy of fluorodeoxyglucose-PET imaging for differentiating benign from malignant pleural effusions: a meta-analysis. Chest 2015;147:502-12. [Crossref] [PubMed]
- Metintas M, Yildirim H, Kaya T, et al. CT Scan-Guided Abrams' Needle Pleural Biopsy versus Ultrasound-Assisted Cutting Needle Pleural Biopsy for Diagnosis in Patients with Pleural Effusion: A Randomized, Controlled Trial. Respiration 2016;91:156-63. [Crossref] [PubMed]
- Heelan RT, Rusch VW, Begg CB, et al. Staging of malignant pleural mesothelioma: comparison of CT and MR imaging. AJR Am J Roentgenol 1999;172:1039-47. [Crossref] [PubMed]
- Copley SJ, Wells AU, Sivakumaran P, et al. Asbestosis and Idiopathic Pulmonary Fibrosis: Comparison of Thin-Section CT Features. Radiology 2003;229:731-6. [Crossref] [PubMed]
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators, Henschke CI, Yankelevitz DF, et al. Survival of Patients with Stage I Lung Cancer Detected on CT Screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Proposed Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). 2014. Available online: http://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=274&bc=AiAAAAAAAgAAAA==&
- Paci E, Puliti D, Lopes Pegna A, et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017;72:825-31. [Crossref] [PubMed]
- Wolff H, Vehmas T, Oksa P, et al. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scand J Work Environ Health 2015;41:5-15. [Crossref] [PubMed]
- Suganuma N, Kusara Y, Hering KG, et al. Reliability of the Proposed International Classification of High-Resolution Computed Tomography for Occupational and Environmental Respiratory Disease. J Occup Health 2009;51:210-22. [Crossref] [PubMed]
- Vierikko T, Järvenpää R, Autti T, et al. Chest CT screening of asbestos-exposed workers: lung lesions and incidental findings. Eur Respir J 2007;29:78-84. [Crossref] [PubMed]
- Roberts HC, Patsios DA, Paul NS, et al. Screening for malignant pleural mesothelioma and lung cancer in individuals with a history of asbestos exposure. J Thorac Oncol 2009;4:620-8. [Crossref] [PubMed]
- Das M, Mühlenbruch G, Mahnken AH, et al. Asbestos Surveillance Program Aachen (ASPA): initial results from baseline screening for lung cancer in asbestos-exposed high-risk individuals using low-dose multidetector-row CT. Eur Radiol 2007;17:1193-9. [Crossref] [PubMed]
- Mastrangelo G, Ballarin MN, Bellini E, et al. Feasibility of a screening programme for lung cancer in former asbestos workers. Occup Med (Lond) 2008;58:175-80. [Crossref] [PubMed]
- Fasola G, Belvedere O, Aita M, et al. Low-dose computed tomography screening for lung cancer and pleural mesothelioma in an asbestos-exposed population: baseline results of a prospective, nonrandomized feasibility trial--an Alpe-adria Thoracic Oncology Multidisciplinary Group Study (ATOM 002). Oncologist 2007;12:1215-24. [Crossref] [PubMed]
- Tiitola M, Kivisaari L, Huuskonen MS, et al. Computed tomography screening for lung cancer in asbestos-exposed workers. Lung Cancer 2002;35:17-22. [Crossref] [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [Crossref] [PubMed]
- Ollier M, Chamoux A, Naughton G, et al. Chest CT scan screening for lung cancer in asbestos occupational exposure. A systematic review and meta-analysis. Chest 2014;145:1339-46. [Crossref] [PubMed]
- Lopes Pegna A, Picozzi G, Mascalchi M, et al. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 2009;64:34-40. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Baseline Characteristics of Participants in the Randomized National Lung Screening Trial. J Natl Cancer Inst 2010;102:1771-9. [Crossref] [PubMed]
- Remy-Jardin M, Sobaszek A, Duhamel A, et al. Asbestos-related pleuropulmonary diseases: evaluation with low-dose four-detector row spiral CT. Radiology 2004;233:182-90. [Crossref] [PubMed]
- Ohar J, Sterling DA, Bleecker E, et al. Changing patterns in asbestos-induced lung disease. Chest 2004;125:744-53. [Crossref] [PubMed]
- Ameille J. The different pleuro-pulmonary pathologies related to asbestos: definitions, epidemiology and evolution. Rev Mal Respir 2012;29:1035-46. [Crossref] [PubMed]
- Nielsen LS, Bælum J, Rasmussen J, et al. Occupational asbestos exposure and lung cancer--a systematic review of the literature. Arch Environ Occup Health 2014;69:191-206. [Crossref] [PubMed]
- McCormack V, Peto J, Byrnes G, et al. Estimating the asbestos-related lung cancer burden from mesothelioma mortality. Br J Cancer 2012;106:575-84. [Crossref] [PubMed]
- Yankelevitz DF, Henschke CI. Does 2-year stability imply that pulmonary nodules are benign? AJR Am J Roentgenol 1997;168:325-8. [Crossref] [PubMed]
- Yankelevitz DF, Gupta R, Zhao B, et al. Small pulmonary nodules: evaluation with repeat CT--preliminary experience. Radiology 1999;212:561-6. [Crossref] [PubMed]
- Yankelevitz DF, Reeves AP, Kostis WJ, et al. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology 2000;217:251-6. [Crossref] [PubMed]


