A novel strategy for cardiopulmonary support during lung transplantation
Introduction
The indications for cardiopulmonary support during lung transplantation include inadequate gas exchange leading to severe hypoxemia or hypercapnia, right ventricle (RV) dysfunction, and pulmonary hypertension (1). Currently, modalities employed for cardiopulmonary support are traditional cardiopulmonary bypass (CPB) or venoarterial extracorporeal membrane oxygenation (VA-ECMO). While both techniques are highly effective, prior reports suggest they may be associated with significant morbidity including severe bleeding, primary graft dysfunction (PGD), stroke, and complications related to arterial cannulation (2,3).
We reasoned that RV bypass combined with extracorporeal membrane oxygenation, using a percutaneous approach, would be sufficient to address the most common indications for cardiopulmonary support during lung transplantation, namely poor gas exchange and RV support, while obviating the inherent risks of CPB and VA-ECMO. We explored the merits of the two percutaneous devices available for RV support, the ProtekDuo (CardiacAssist, Inc., Pittsburgh, PA, USA) dual-lumen cannula (DLC) and the Impella RP (Abiomed, Inc., Danvers, MA, USA), and selected the former since it can be connected to an ECMO circuit. When used in a right atrial-pulmonary artery configuration (Figure 1), deoxygenated blood is drained from the right atrium and oxygenated blood is returned into the main pulmonary artery (PA), bypassing and unloading the RV.
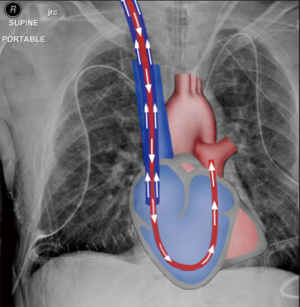
Case presentation
We employed this technique in a 55-year-old male who underwent bilateral lung transplantation for mixed emphysema and pulmonary fibrosis. He suffered from severe hypoxemia and pulmonary hypertension with mean PA pressure of 66 mmHg. Further, he had a moderately dilated RV with septal flattening and reduced systolic function on echocardiogram. Thus, he required intraoperative cardiopulmonary support which was initiated at the time of transplant. A 29-F DLC was placed percutaneously through the right internal jugular vein and positioned using fluoroscopy (Figure 2). We used a ROTAFLOW centrifugal pump and QUADROX oxygenator (MAQUET Cardiovascular, LLC, Wayne, NJ, USA) with this circuit. The RV was effectively decompressed throughout the case with ECMO flows over 4 liters/minute despite elevated pulmonary pressures. Bilateral sequential lung transplantation was performed without intraoperative complications. Total ischemic times for the left and right lung were 336 and 416 minutes, respectively. The DLC was left in place postoperatively to provide ongoing RV support and the patient was decannulated at bedside on post-operative day (POD) 2. Postoperative echocardiogram revealed normalization of RV size and systolic function. We previously reported that venovenous ECMO can be successfully performed without anticoagulation (4). Similarly, in this case, we did not use any heparin during cardiopulmonary support. Recovery was uncomplicated and the patient was discharged home on POD 18. A CT pulmonary angiography revealed no embolism resulting from the DLC. He remains breathing room air at eight months with an FEV1 of 73% predicted.
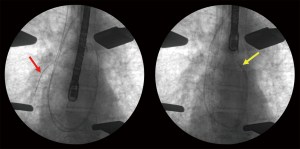
Discussion
In this report, we demonstrated the feasibility of a novel percutaneous strategy of RV bypass coupled with membrane oxygenation using a single, peripherally inserted venous DLC that mitigates the inherent risks of traditional cardiopulmonary support techniques. As demonstrated in this case, the cannula can be left in place postoperatively for ongoing RV support or in the case of PGD development after transplantation. This is a substantial advantage over the alternative central RA to PA cannulation. Furthermore, the flows achieved through the 29-F cannula provided adequate cardiopulmonary support throughout the case. Since bronchial arteries are not routinely re-anastomosed during lung transplantation, lung allografts are dependent on pulmonary blood flow and may incur a risk of ischemia or vascular thrombosis if pulmonary blood flow is completely diverted (5). The DLC-RV bypass configuration we used delivers oxygenated blood to the allograft and minimizes the risk of allograft ischemia. The lack of arterial cannulation may reduce complications and further enhance patient comfort and mobility. Further, since it only requires venous access, bedside decannulation is possible. In the standard configurations of venovenous ECMO, oxygenated blood is returned to the right atrium which makes it unsuitable in cases of a failing RV or severe pulmonary hypertension. Our novel DLC approach delivers oxygenated blood flow directly into the main PA, thereby bypassing the RV and overcoming RV failure, pulmonary hypertension, and hypoxemia. While the DLC itself does not induce pulmonary regurgitation with a competent pulmonary valve, we caution against using this technique in patients with pulmonic valve insufficiency at baseline as the degree of regurgitation could worsen with pressurized PA flows, leading to RV distension. Placement of this cannula does not preclude going on full CPB or central VA-ECMO. If need arises for full cardiopulmonary support, both cannula lumens (right atrial and PA) can be used as venous drainage and an arterial cannula can be placed into the aorta. Later, the arterial cannula can be removed and the DLC converted back to the standard configuration if ongoing respiratory or RV support is required.
In summary, our strategy of RV bypass with membrane oxygenation can provide adequate and safe cardiopulmonary support during lung transplantation. Unique benefits of this approach include a lack of systemic heparinization and arterial cannulation that mitigate the inherent risks of traditional CPB and VA-ECMO techniques, as well as percutaneous venous access allowing for bedside decannulation. This novel approach represents a useful addition to the armamentarium for CPB techniques in lung transplantation. Further studies should be performed to determine the efficacy and safety of this technique compared to the traditional approaches.
Acknowledgements
We thank Ms. Elena Susan for formatting and submitting this manuscript.
Funding: This work was supported by the National Institutes of Health (HL125940 to A Bharat, T32 DK077662 to R Fernandez).
Footnote
Conflicts of Interest: A Bharat is a consultant for Cardiac Assist, Inc. The other authors have no conflicts of interest to declare.
Informed Consent: Witten informed consent was obtained from the patient for the writing of this manuscript.
References
- Ius F, Kuehn C, Tudorache I, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg 2012;144:1510-6. [Crossref] [PubMed]
- Ramchandani M, Al Jabbari O, Abu Saleh WK, et al. Cannulation Strategies and Pitfalls in Minimally Invasive Cardiac Surgery. Methodist Debakey Cardiovasc J 2016;12:10-3. [Crossref] [PubMed]
- Yu WS, Paik HC, Haam SJ, et al. Transition to routine use of venoarterial extracorporeal oxygenation during lung transplantation could improve early outcomes. J Thorac Dis 2016;8:1712-20. [Crossref] [PubMed]
- Tomasko J, Prasad SM, Dell DO, et al. Therapeutic anticoagulation-free extracorporeal membrane oxygenation as a bridge to lung transplantation. J Heart Lung Transplant 2016;35:947-8. [Crossref] [PubMed]
- Burns KE, Iacono AT. Pulmonary embolism on postmortem examination: an under-recognized complication in lung-transplant recipients? Transplantation 2004;77:692-8. [Crossref] [PubMed]


