Early and mid-term haemodynamic performance and clinical outcomes of St. Jude Medical Trifecta™ valve
Introduction
Aortic valve replacement (AVR) surgery has become a safe and reproducible procedure and is the gold standard for the treatment of severe symptomatic aortic valve disease (AVD) (1). Aortic bioprostheses use is growing as the age of aortic disease patients increases (2,3). The low thrombogenic risk improved haemodynamic performance and longer durability, of recent pericardial bioprosthesis models (that translated into promising clinical outcomes) have made them an appealing solution even for younger patients. Trifecta prosthesis (St. Jude Medical, Inc., St. Paul, MN, USA) is an aortic pericardial bioprosthesis with a titanium stent, designed to have minimal haemodynamic impact [smaller transprosthetic gradients and increased effective orifice area (EOA)]; leaflets are mounted as a single pericardial patch in the outer aspect of the struts which allows for almost circular cross-section during systole (4,5). Trifecta bioprosthesis received European CE-mark approval in 2010 and Food and Drug Administration (FDA) approval in 2011 (4). Other reports have demonstrated excellent clinical results, favourable haemodynamic profile, as well as positive ventricular remodelling and mass regression (6-9), but large clinical registries with longer follow-up are lacking.
In this study we report in-hospital clinical results, early ambulatory haemodynamic profile and mid-term clinical outcomes of Trifecta bioprosthesis implanted at our centre during a 5-year period.
Methods
Study design and patients
We present a retrospective, observational and descriptive study. All individuals who underwent AVR with Trifecta bioprosthesis, between 1st July of 2011 and 30th June of 2016 at the Cardiothoracic Surgery Department of Centro Hospitalar São João, were included. Both patients with isolated AVR and other concomitant procedures were included and no exclusion criteria were applied.
Surgical technique
Patient selection for these specific aortic valves was left at surgeons’ discretion and was not study-related. All aortic bioprostheses were implanted in a supra-annular position under mild hypothermic or normothermic cardiopulmonary bypass (CPB) and cardioplegic arrest. The valves were sutured using interrupted U-shaped pledgeted 2-0 polyester stitches, interrupted simple 4-0 polyester sutures or continuous polypropylene suture, again, according to surgeons’ preference.
Study setting and variables
Clinical and surgical information regarding preoperative and postoperative periods were retrospectively collected through clinical files. Echocardiographic evaluation data were obtained from the local database. According to centre protocol, patients were evaluated for postoperative clinical observation and transthoracic echocardiography (TTE) at 4±3 months. From this evaluation, we obtained mean gradients, EOA, as also the presence of patient-prosthesis mismatch (PPM): inadequate prosthetic EOA index (EOAi) to the patient’s body surface area (BSA). PPM was defined as moderate (0.65 cm2/m2 ≤ EOAi <0.85 cm2/m2) or severe (EOAi <0.65 cm2/m2) (10). The type of procedure was classified as elective (patients who were routinely admitted for surgery), urgent (patients who have not been electively admitted for surgery but required a definitive procedure before discharge) and emergent (patients requiring intervention before the next working day).
Mortality and valve-related re-intervention were censored by accessing the National Registry and local clinical files, respectively, in February 2017 (mean follow-up time of 27±17 months, maximum of 67 months).
Immediate postoperative events considered were: de novo atrial fibrillation (AF) episodes, permanent pacemaker implantation, renal function impairment (double or greater increase in serum creatinine relative to baseline value or need of dialysis), prolonged invasive ventilation (mechanical ventilation >24 h), severe thrombocytopenia (platelet count <30×109/L), stroke, length of hospital stay, early chest re-exploration for bleeding or tamponade and mortality at 30 days. Structural valve deterioration (SVD) was considered if any intrinsic changes in the valve occurred. A non-structural valve dysfunction (NSVD) was defined as any abnormality not intrinsic to the implanted valve that did not directly involved valve components, including also new onset of coronary ischemia from coronary ostial obstruction (11).
Anticoagulation therapy
Patients under 80 years of age without contraindication were routinely discharged on Vitamin K antagonist (target INR of 2.0–3.0) for 3 months after AVR, unless continuation was required for another reason. Older patients (≥80 years) received oral acetylsalicylic acid (100 to 150 mg) instead of vitamin K antagonist if they were not on oral anticoagulation therapy for other reason.
Statistical analysis and ethics
Data processing and statistical analysis were done in Statistical Package for the Social Sciences version 21 (SPSS) Software, IBM Corporation, Armonk, NY, USA, for Windows. Normality distribution inspection was performed through visual analysis of histograms of the total sample. Continuous variables are presented by means and standard deviation or by median, minimum and maximum, as adequate. Categorical variables are presented by absolute values and relative frequencies (valid percentage, excluding missing values). Comparisons between patients with multiple versus isolated procedures were done using Student’s t test or Mann-Whitney test for continuous variables. Comparison between pre- and post-operative functional class by New York Heart Association (NYHA) classification was performed with Wilcoxon test for paired variables. Kaplan-Meier curves were used to evaluate time-to-event data, specifically cumulative survival and freedom from reoperation. A descriptive and inferential statistical analysis was carried out with a level of significance of 0.05. This study was approved by the local Ethics Committee in June 2016 (Institutional Review Board 143-16). Patient informed consent was waived since the study was retrospective and observational. The confidentiality and anonymity of the identification data were respected following the guidelines emanated from the Declaration of Helsinki of 1964, revised in Fortaleza, in 2013.
Results
Demographics
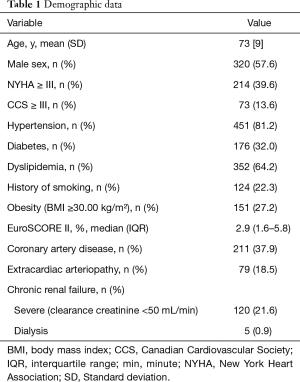
We included 556 patients with mean age of 73±9 years (20 to 91 years) and 57.6% were male. The median European System for Cardiac Operative Risk Evaluation (EuroSCORE) II in overall sample was 2.9 (interquartile range, 1.6–5.8) being the mean 5.2 (Table 1). Considering isolated AVR, the median EuroSCORE II was 1.8 (interquartile range, 1.2–3.1) and in patients with concomitant procedures was significantly higher: 4.2 (interquartile range, 2.5–9.1), P<0.001.
Full table
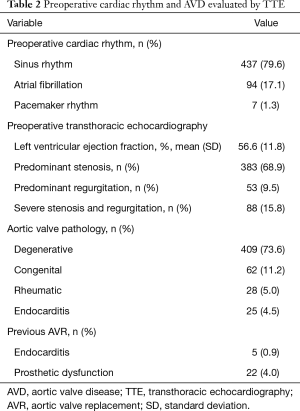
Of the 556 patients who underwent AVR, 529 (95.1%) presented native AVD namely stenosis (68.9%), congenital defect (11.2%) and regurgitation (9.5%). Moreover, 30 (5.4%) patients had endocarditis of which 25 (4.5%) occurred in native valves and 5 (0.9%) in previously implanted prosthesis and 22 (4.0%) had a previously implanted dysfunctional aortic prosthesis (Table 2).
Full table
Surgical
Most procedures were elective 420 (75.5%), 128 (23.0%) were urgent and 8 (1.4%) emergent. Isolated AVR was performed in 255 (45.9%) patients and 301 (54.1%) underwent concomitant procedures: coronary artery bypass grafting (CABG) in 170 (30.6%), intervention in other valves was performed in 80 (14.5%), ascending aorta replacement in 34 (6.1%) which included 14 (2.5%) Bentall operations. Mean CPB and cross clamp times in multiple procedures were, as expected, longer than in isolated AVR: 156±58 vs. 97±32 minutes (P<0.001) and 108±40 vs. 69 ± 23 minutes (P<0.001), respectively (Table 3).
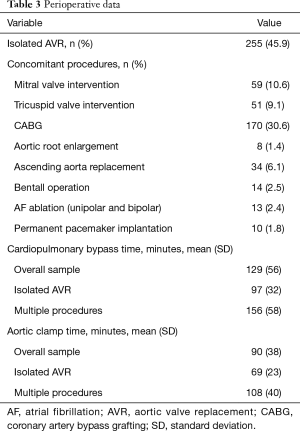
Full table
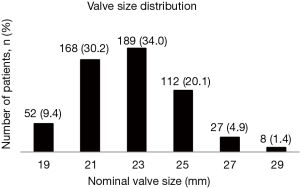
The 23-mm diameter was the most frequently implanted size followed by the 21-mm diameter (Figure 1).
Early outcomes
The median length of hospital stay was 8 days (5 to 115 days). During the immediate postoperative period, de novo AF occurred in 179 (32.2%) individuals, 74 (13.6%) required prolonged ventilation and 21 (3.8%) suffered a clinically detected stroke confirmed by CT scan. Worsening of renal function occurred in 28 (5.1%) subjects and severe thrombocytopenia in 17 (3.1%) patients. Complete heart block and AF with slow ventricular response led to the implantation of permanent pacemaker in 20 (3.7%) and 3 (0.6%) patients, respectively.
Twenty-six patients underwent early reoperation (≤30 days post-implant): 12 (2.1%) for chest re-exploration due to bleeding (1 patient also required CABG due to left main coronary ostial occlusion—NSVD); 3 (0.5%) patients presented NSVD of which 2 underwent CABG due to right coronary ostium obstruction and the other had stent distortion mandating a new AVR; 1 (0.2%) required CABG for coronary artery disease that was deferred and 10 (1.8%) were reoperated due to others causes (Table 4).
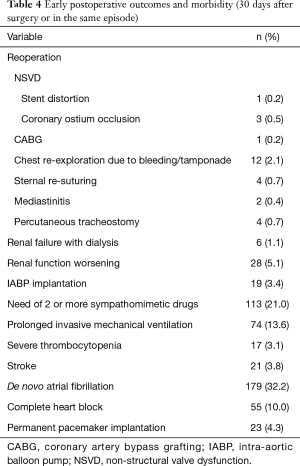
Full table
Intra-operative mortality was 0.5% (n=3), none attributable to valve causes (one due to hemorrhagic shock, other due to ventricular laceration during chest re-entry in a reoperation and the last due to uncontrollable coagulopathy associated with low cardiac output). Overall 30-day mortality was 5.4% (n=30), including intra-operative mortality. Deceased patients had a higher mean EuroSCORE II (10.3±9.5) than survivors (4.9±6.8, P<0.001). Early mortality causes were cardiac in 20 (66.7%) and infection in 10 (33.3%) patients. No valve thrombosis or clinically significant haemolysis was registered in the immediate postoperative period.
Follow-up echocardiographic data
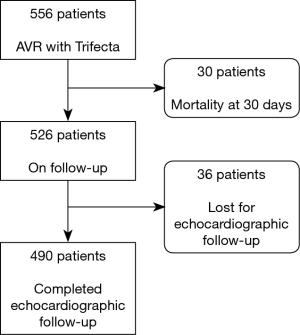
Follow-up echocardiogram was performed in 490 of the 526 patients (6.8% lost to follow-up), as shown in Figure 2.
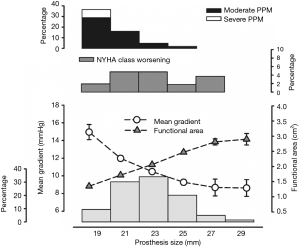
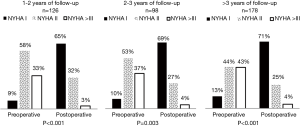
The mean transprosthetic gradient (MTG) was 10.9±4.1 mmHg and EOA was 2.0±0.5 cm2. Figure 3 represents MTG, EOA, PPM and NYHA functional class worsening by prosthesis size. Moderate PPM occurred in 52 (11.3%) and severe PPM in 5 (1.1%) individuals.
Thirty-one (6.3%) patients had trivial intraprosthetic regurgitation, 26 (5.3%) had mild and only one patient presented moderate regurgitation; 9 (1.8%) a periprosthetic leak: 1 trivial, 5 of mild degree and 3 moderates.
Mid-term outcomes
During a median follow-up time of 27±17 months, we registered 5 bioprosthesis related re-interventions: 4 for endocarditis and 1 for NSVD caused by partial dehiscence of the implantation suture. Kaplan-Meier analysis showed that freedom from valve-related reoperation was 99.4% at 1 year, 98.8% at 2 and 3 years, 97.6% at 4 years and 92.7% from year 5 until the end of follow-up period. Other events which required re-hospitalization are presented in Table 5.
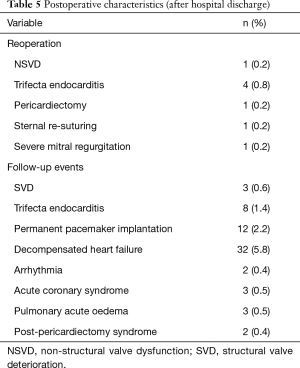
Full table
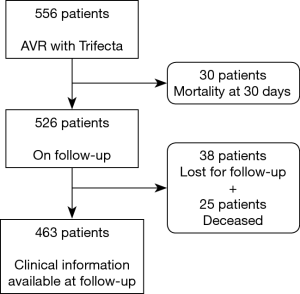
Considering all surviving patients at 30 days (n=526) who were followed by a referral Cardiologist (n=463, 12.0% lost to follow-up) presented in Figure 4, we identified 3 patients with SVD during follow-up period. One patient presented a moderate to severe intraprosthetic regurgitation at 41 months and was not reoperated due to a terminal pulmonary neoplasia. The others patients presented a moderate intraprosthetic regurgitation at 13 and 67 months, respectively.
Of notice, from all 463 patients who underwent clinical evaluation only 402 individuals had paired data of pre- and post-operative NYHA functional class assessment. Of these, 126 completed 1 to 2 years of follow-up, 98 completed 2 to 3 years and 178 fulfilled 3 or more years. Patients with a follow-up period lower than 1 year of follow-up were excluded. According to Figure 5, patients’ clinical status significantly improved in all cases and the majority of patients were in class I.
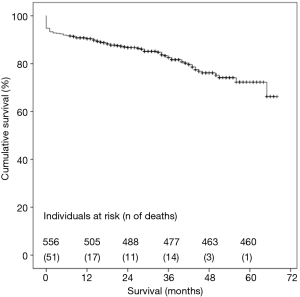
Considering all-causes mortality, cumulative survival at 1-, 3- and 5-year was 90.8%, 83.3% and 72.3%, respectively, as shown in Figure 6. There were no differences in cumulative survival when comparing multiple procedures and isolated AVR (Log Rank test, P=0.69).
Discussion
This retrospective study reports the experience with St. Jude Medical Trifecta valve of a tertiary single-centre over 5 years. Good haemodynamic performance and safety profile of this bioprosthesis were ascertained.
More than three decades after the introduction of modern prosthesis, the choice for the perfect aortic valve remains controversial. Still, the clinical decision becomes increasingly challenging with the rise of life expectancy, the presence of a large spectrum of comorbidities and the good haemodynamic performance presented by transcatheter aortic valves.
The search for a biological prosthesis with almost physiological EOA and the smallest residual transvalvular pressure gradients was on the basis of the development of the innovative pericardium bioprostheses (4,7,12). Also, stentless bioprosthesis aimed to achieve a more physiological flow (13,14) and it was expected that the emergence of these valves would lead to a decrease in the use of stented bioprosthesis, which so far did not occur (15).
The easier implantation technique combined with optimal haemodynamic performance of the latest generation of stented bioprosthesis might have reduce the use of stentless valves (4,7,8), which require a technically more complex implantation and a longer learning curve (5,7,15-17). Although this is not a comparative study, our results support this hypothesis. The easy surgical technique, allowed all surgeons in the department to implant the Trifecta bioprosthesis, with good haemodynamic results (10.9±4.1 mmHg and 2.0±0.5 cm2, MTG and EOA, respectively) and a low incidence of leaks: only 3 (0.6%) patients had a moderate periprosthetic leak at 4±3 months of follow-up. This low incidence might be due to the narrower sewing ring which is contoured by silicone inside, allowing a better apposition on the native annulus (7,14).
To ensure adequate prosthesis size, some surgeons perform aortic root enlargement. We only registered 8 (1.4%) patients, similar to Goldman et al. and Deutsch et al. studies who reported this procedure in 2.0% and 1.6% patients, respectively (6,7). When comparing the Trifecta with the Perimount Magna and Perimount Magna Ease bioprostheses, Wendt et al. observed a lower use of aortic root enlargement with Trifecta (9.1%, vs. 25.4% and 12.1%, respectively), which they hypothesized it could be due to larger aortic valve areas provided by Trifecta in comparison with other stented bioprostheses (18). Interestingly, Yadlapati et al. did not perform any aortic root enlargement during AVR in their series of patients that received a Trifecta implant (19).
With regard to the haemodynamic profile of Trifecta bioprosthesis, Deutsch et al. showed a MTG, before discharge, for the 19-mm and 21-mm prostheses sizes, of 14.3 and 12.9 mmHg, respectively (7). A systematic review and meta-analysis by Phan et al. revealed a MTG of 10.7 mmHg for the 19-mm prosthesis (8). Bavaria et al. also demonstrated an excellent haemodynamic performance in 1014 patients enrolled at 31 centres, documenting a MTG, at hospital discharge, of 9.3 mmHg in the 19-mm diameter valve (4). Our results are in line with these reports, as we observed 15.0 mmHg in 19-mm and 12.0 mmHg in 21-mm Trifecta valve.
In comparison with other stented bioprosthesis Trifecta showed better haemodynamic performance than the Perimount Magna Ease (20), the Mitroflow and the Perimount Magna (21), manifested in the latter study by lower MTG, higher EOA and EOAi, as well as, lower incidence of severe PPM. Likewise, we also observed a low incidence of moderate (11.3%) and severe (1.1%) PPM.
PPM has been associated with worse outcomes after AVR surgery (22), as manifest by increased all-cause and cardiac-related mortality (23), longer stay in the intensive care unit (24), less regression of left ventricle mass (25) and more neurologic events (26). It is also an independent predictor of SVD (27). We observed severe PPM in only 1.1% individuals (MTG of 14±4.8 mmHg), a much lower incidence when compared with the 9.8% reported in a large meta-analysis including more than 27,000 patient that underwent AVR surgery (23).
Mortality at 30 days ranged from 1.5% to 3.8% in other Trifecta series (6,28). Our cohort presented a higher 30-day mortality rate (5.4%). Yet, we found higher mortality rate comparing to other studies, at follow-up. Indeed, Bavaria et al. (4) and Goldman et al. (6) reported a cumulative survival of 95.8% at 1 year and 93.0% at 3 years and in the present study, these values were 90.8% and 83.3%, respectively. This difference in 30-day mortality and cumulative survival values, might be related to the higher mean EuroSCORE II of our sample—4.9%, reaching 10.3% in early mortality individuals. In fact, we included all patients that underwent AVR with Trifecta bioprosthesis apart of their comorbidities or concomitant procedures. In contrast, Goldman et al. and Bavaria et al. (4,6) excluded patients with active endocarditis, renal dialysis, significant cardiovascular abnormalities such as aortic dissection, life expectancy less than 2 years and patients requiring concomitant replacement of another valve. Indeed, our series includes a large proportion of patients with high surgical risk: 21.6% patients had severe chronic renal failure (5 patients in dialysis), poor functional class (39.6% individuals had NYHA ≥III), 4.7% (26) presented active endocarditis, 24.4% underwent urgent or emergent surgery, 54.1% had concomitant procedures, 4.9% (27) had a previously implanted aortic valve prosthesis, 1 patient had aortic dissection and 6 patients presented with cardiogenic shock at admission.
Freedom from reoperation was also similar being 99.6% at 1 year, 99.4% at 2 and 98.6% at 3-year in our study; 99.4% at 1 year and 98.6% at 3 years in the study of Goldman et al. (6) and, finally, 99.4% at 2 years in the study of Bavaria et al. (4). None of our cases of reoperation was due to SVD, while Goldman presented 11 cases more than 30 days after implantation (6).
However, one of our reoperations that required Trifecta prosthesis replacement 12 days after implantation was due to stent distortion that caused severe intraprosthetic regurgitation. This condition could be attributed to an inappropriate sizing or an incorrect annular decalcification that might reduce haemodynamic performance and potentially cause regurgitation of improperly approximating cusps (5,29). Same authors emphasize the importance of the implant technique to prevent stent distortion which can abolish the benefits of the cuff designed to conform to the native annulus after implantation (8,30,31).
Lower coronary ostia and prosthesis oversizing can lead to coronary ostial obstruction or occlusion (29). We registered 3 (0.5%) cases of myocardial ischemia due to coronary obstruction causing ventricular dysfunction at early postoperative period that required CABG in patients without significant coronary artery disease.
Study limitations
This study has limitations related to its retrospective design; data in some variables was absent and some patients were lost to follow-up. Moreover, this is a single-centre study prone to selection bias as the choice of the prosthesis was left to surgeon’s preference. Preoperative, discharge and follow-up echocardiographic data were not available in all patients and echocardiographic evaluation was not performed by the same physician and with the same equipment. A longer follow-up period is necessary to evaluate prosthesis failure and valve-related adverse events. Finally, we only evaluated all-causes mortality and it would be important to determine cardiac-related deaths as well as other major adverse cardiovascular and cerebrovascular events during follow-up.
Conclusions
We performed a descriptive analysis of the Trifecta bioprosthesis including 556 patients. Our findings show good haemodynamic performance and favourable clinical outcomes with this bioprosthesis. Considering the bioprosthesis’ recent market introduction, mid and long-term follow-up data are crucial to determine its durability, efficacy and safety outcomes.
Acknowledgements
Funding: This work was supported by DOCnet Project (NORTE-01-0145-FEDER-000003), supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF); European Structural and Investment Funds (ESIF), under Lisbon Portugal Regional Operational Programme and National Funds through FCT - Foundation for Science and Technology under project POCI-01-0145-FEDER-016385; Faculty of Medicine, University of Porto and FSE–Fundo Social Europeu through Norte Portugal Regional Operational Programme (NORTE 2020: 08-53-69-FSE-000024) - doctoral programs.”
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the local Ethics Committee in June 2016 (Institutional Review Board 143-16). Patient informed consent was waived since the study was retrospective and observational.
References
- Genereux P, Stone GW, O'Gara PT, et al. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol 2016;67:2263-88. [Crossref] [PubMed]
- Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med 2014;371:744-56. [Crossref] [PubMed]
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Bavaria JE, Desai ND, Cheung A, et al. The St Jude Medical Trifecta aortic pericardial valve: results from a global, multicenter, prospective clinical study. J Thorac Cardiovasc Surg 2014;147:590-7. [Crossref] [PubMed]
- Mariscalco G, Mariani S, Bichi S, et al. St. Jude Medical Trifecta aortic valve: results from a prospective regional multicentre registry. J Cardiothorac Surg 2015;10:169. [Crossref] [PubMed]
- Goldman S, Cheung A, Bavaria JE, et al. Midterm, multicenter clinical and hemodynamic results for the Trifecta aortic pericardial valve. J Thorac Cardiovasc Surg 2017;153:561-9.e2. [Crossref] [PubMed]
- Deutsch MA, Prinzing A, Fiegl K, et al. Early haemodynamic performance of a latest generation supra-annular aortic bioprosthesis: experience from a large single-centre series. Eur J Cardiothorac Surg 2016;49:1691-8. [Crossref] [PubMed]
- Phan K, Ha H, Phan S, et al. Early hemodynamic performance of the third generation St Jude Trifecta aortic prosthesis: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2015;149:1567-75.e1-2.
- Rubens FD, Gee YY, Ngu JM, et al. Effect of aortic pericardial valve choice on outcomes and left ventricular mass regression in patients with left ventricular hypertrophy. J Thorac Cardiovasc Surg 2016;152:1291-8.e2. [Crossref] [PubMed]
- Pibarot P, Dumesnil JG. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol 2000;36:1131-41. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). G Ital Cardiol (Rome) 2013;14:167-214. [PubMed]
- Dell'Aquila AM, Schlarb D, Schneider SR, et al. Clinical and echocardiographic outcomes after implantation of the Trifecta aortic bioprosthesis: an initial single-centre experience. Interact Cardiovasc Thorac Surg 2013;16:112-5. [Crossref] [PubMed]
- D'Onofrio A, Cresce GD, Bolgan I, et al. Clinical and hemodynamic outcomes after aortic valve replacement with stented and stentless pericardial xenografts: a propensity-matched analysis. J Heart Valve Dis 2011;20:319-25; discussion 26. [PubMed]
- Ali A, Halstead JC, Cafferty F, et al. Are stentless valves superior to modern stented valves? A prospective randomized trial. Circulation 2006;114:I535-40. [Crossref] [PubMed]
- Funder JA. Current status on stentless aortic bioprosthesis: a clinical and experimental perspective. Eur J Cardiothorac Surg 2012;41:790-9. [Crossref] [PubMed]
- Cheng D, Pepper J, Martin J, et al. Stentless versus stented bioprosthetic aortic valves: a systematic review and meta-analysis of controlled trials. Innovations (Phila) 2009;4:61-73. [Crossref] [PubMed]
- Pepper J, Cheng D, Stanbridge R, et al. Stentless Versus Stented Bioprosthetic Aortic Valves: A Consensus Statement of the International Society of Minimally Invasive Cardiothoracic Surgery (ISMICS) 2008. Innovations (Phila) 2009;4:49-60. [Crossref] [PubMed]
- Wendt D, Thielmann M, Plicht B, et al. The new St Jude Trifecta versus Carpentier-Edwards Perimount Magna and Magna Ease aortic bioprosthesis: is there a hemodynamic superiority? J Thorac Cardiovasc Surg 2014;147:1553-60. [Crossref] [PubMed]
- Yadlapati A, Diep J, Barnes MJ, et al. Comprehensive hemodynamic performance and frequency of patient-prosthesis mismatch of the St. Jude Medical Trifecta bioprosthetic aortic valve. J Heart Valve Dis 2014;23:516-23. [PubMed]
- Bach DS, Patel HJ, Kolias TJ, et al. Randomized comparison of exercise haemodynamics of Freestyle, Magna Ease and Trifecta bioprostheses after aortic valve replacement for severe aortic stenosis. Eur J Cardiothorac Surg 2016;50:361-7. [Crossref] [PubMed]
- Ugur M, Suri RM, Daly RC, et al. Comparison of early hemodynamic performance of 3 aortic valve bioprostheses. J Thorac Cardiovasc Surg 2014;148:1940-6. [Crossref] [PubMed]
- Popma JJ, Khabbaz K. Prosthesis-patient mismatch after "high-risk" aortic valve replacement. J Am Coll Cardiol 2014;64:1335-8. [Crossref] [PubMed]
- Head SJ, Mokhles MM, Osnabrugge RL, et al. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur Heart J 2012;33:1518-29. [Crossref] [PubMed]
- Astudillo LM, Santana O, Urbandt PA, et al. Clinical predictors of prosthesis-patient mismatch after aortic valve replacement for aortic stenosis. Clinics (Sao Paulo) 2012;67:55-60. [Crossref] [PubMed]
- Kandler K, Møller CH, Hassager C, et al. Patient-prosthesis mismatch and reduction in left ventricular mass after aortic valve replacement. Ann Thorac Surg 2013;96:66-71. [Crossref] [PubMed]
- Nozohoor S, Nilsson J, Lührs C, et al. The influence of patient-prosthesis mismatch on in-hospital complications and early mortality after aortic valve replacement. J Heart Valve Dis 2007;16:475-82. [PubMed]
- Flameng W, Rega F, Vercalsteren M, et al. Antimineralization treatment and patient-prosthesis mismatch are major determinants of the onset and incidence of structural valve degeneration in bioprosthetic heart valves. J Thorac Cardiovasc Surg 2014;147:1219-24. [Crossref] [PubMed]
- Anselmi A, Ruggieri VG, Lelong B, et al. Mid-term durability of the Trifecta bioprosthesis for aortic valve replacement. J Thorac Cardiovasc Surg 2017;153:21-8.e1. [Crossref] [PubMed]
- Modi A, Budra M, Miskolczi S, et al. Hemodynamic performance of Trifecta: single-center experience of 400 patients. Asian Cardiovasc Thorac Ann 2015;23:140-5. [Crossref] [PubMed]
- Permanyer E, Estigarribia AJ, Ysasi A, et al. St. Jude Medical Trifecta aortic valve perioperative performance in 200 patients. Interact Cardiovasc Thorac Surg 2013;17:669-72. [Crossref] [PubMed]
- Jamieson WR. St Jude Medical Trifecta aortic prosthesis: Considerations for implantation. J Thorac Cardiovasc Surg 2015;149:1576-7. [Crossref] [PubMed]