Early and mid-term follow-up of patients receiving arterial switch operation: a single-center experience
Introduction
Since its inception in 1975 by Jatene and colleagues (1), the arterial switch operation (ASO) has become the preferred method for surgical correction of transposition of the great arteries (TGA) and Taussig-Bing anomaly. Although the current operation mortality is low and the late results are satisfactory (2,3), there are still some complications influencing the outcomes of the procedure. The reported common complications include neoaortic root dilation and regurgitation, neopulmonary stenosis and obstructive coronary disease (4,5).
Transfer of the coronary artery origins is key to a successful ASO. Coronary artery reimplantation to the neoaorta has been performed by different techniques including various shaped button techniques and the trap door technique (6). In our department of Wuhan Union Hospital, we have employed split incision for coronary reimplantation. This technique can alleviate the coronary artery tension and avoid coronary artery distortion or obstruction while it also enlarges the neoaortic root and increases the risk of aortic regurgitation. The classical technique for pulmonary artery reconstruction is single pantaloon pericardial patch technique. Reported augmentation techniques for the neopulmonary artery include the direct anastomosis and extended pericardial patch technique (7,8). We have employed extended fresh pericardial patch for neopulmonary artery reconstruction. The purpose of this study was to investigate early and mid-term outcomes using our reconstruction and reimplantation techniques.
Methods
Study population
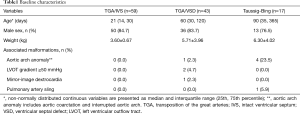
We conducted a retrospective review of patients who underwent ASO with the diagnosis of d-TGA or Taussig-Bing anomaly at Wuhan Union Hospital between January 2007 and December 2013. A total of 119 consecutive patients were included in the study. Patients with various forms of TGA undergoing palliative ASO, double-switch operation, and Nikaidoh procedure were excluded from this study. The median age at operation was 30 days (range, 1 day–8 years). Among them, 13 (10.9%) patients were older than 6 months. 8 (6.7%) patients were older than 12 months. The median weight was 3.8 kg (range, 2.0–23.0 kg). Patient characteristics are depicted in Table 1. The ventricular septum was intact in 59 (49.6%) patients, 43 (36.1%) had ventricular septal defect, and 17 (14.3%) had a Taussig-Bing anomaly, 5 (4.2%) patients had aortic arch anomalies, 2 (1.7%) had a left ventricular outflow tract gradient ≥50 mmHg, 1 (0.8%) had mirror-image dextrocardia and 1 (0.8%) had pulmonary artery sling. The study was conducted in accordance with guidelines from the Committee on Clinical Investigation and approved by the institutional review board.
Full table
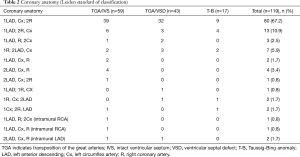
According to the Leiden classification (9), the majority of patients had 1LCx-2R pattern. Ten (8.4%) patients had coronary arteries that originated from a single sinus and 4 (3.4%) patients had intramural coronary artery anatomy. The coronary anatomy of the patients was shown in Table 2.
Full table
Surgical technique
Surgery was performed via a median sternotomy under standard moderate hypothermic cardiopulmonary bypass (CPB) with cold cardioplegic arrest. Before initiation of CPB, the aorta was dissected from aortic root to aortic arch, the main pulmonary artery and branch pulmonary arteries were dissected free to the hilum of the lung, allowing optimal mobility after the LeCompte maneuver. Mean pulmonary artery pressure (mPAP) was measured by using a needle inserted into the root of the pulmonary artery and connected to a pressure transmitter and piezometer. After myocardial arrest with cold blood cardioplegia, the ductus arteriosus was cut and sutured, the intracardiac anomaly was corrected. For patients of TGA/ventricular septal defect (TGA/VSD) and Taussig-Bing anomaly with severe pulmonary hypertension (PH), partial closure of VSD with a fenestrated patch was applied with subsequent closure of the remaining defect by surgical or interventional methods. Then the aorta and pulmonary arteries were divided, and the coronary arteries were removed to the aorta using split incision. Specially, make a vertical incision (not a button) in proximal pulmonary artery (neo-aortic root), replant the coronary arteries directly. A LeCompte maneuver was then performed. The distal ascending aorta was anastomosed to the neo-aortic root. With the aortic clamp off, the neopulmonary sinus defects were repaired with two individual autologous pericardial patches, extended posteriorly to lengthen the pulmonary artery. No patients underwent a preliminary pulmonary artery banding procedure in this study.
Postoperative management
Arterial blood pressure and central venous pressure were continuously monitored on a routine basis, and transcutaneous blood oxygen saturation and surface electrocardiogram were measured. Inotropic agents and nitroglycerin were routinely used to maintain adequate arterial blood pressure and prevent coronary artery spasm. All infants were sedated with fentanyl, pipecuronium bromide, and midazolam. Patients with PH were given prolonged ventilation support (more than 48 hours) and intravenous prostaglandin E1. Iloprost was also given by atomization inhalation for severe PH. If mean pulmonary arterial pressure estimated by echocardiography still remains moderate or even severe, oral sildenafil or bosentan was administered. A few patients with persistent PH after discharge were administered with oral sildenafil or bosentan for months or even years and followed up using echocardiography to estimate PH.
Follow-up
Eligible candidates were identified by searching institutional databases in the Departments of Cardiac Surgery. Inpatient chart review allowed description of perioperative events. Patient follow-up and need for reintervention were determined from outpatient clinic charts. Our primary end point was in-hospital deaths, secondary end points were postoperative valve dysfunction, pulmonary artery stenosis and need for reoperation. Patients were contacted by telephone and echocardiography was performed. Postoperative echocardiograms were analyzed for the valve function and neo-pulmonary artery, especially for the incidence and severity of aortic insufficiency and pulmonary stenosis. The gradient of aortic regurgitation was classified as either trace, mild, moderate, or severe depending on the ratio of the width of the regurgitant jet to the diameter of the left ventricular outflow tract (10). The gradient of tricuspid regurgitation and pulmonary regurgitation were also classified as either trace, mild, moderate, or severe depending on Doppler findings. In addition, neopulmonary stenosis was classified depending on Doppler findings as trivial (transpulmonary peak gradient <25 mmHg), mild (25 to 49 mmHg), moderate (50 to 79 mmHg), or severe (>79 mmHg) (11). Echocardiography were performed perioperatively, before discharge, and then 3 or 6, and 12 months post-operatively, and annually thereafter.
Statistical analysis
Continuous variables are summarized by mean ± standard deviation or median and interquartile range (IQR: 25th, 75th percentile) depending on normality of distribution. Categorical variables are represented by frequencies and percentages. Baseline comparisons between patients with D-TGA and intact ventricular septum, ventricular septal defect, and Taussig-Bing anomaly were performed by one-way analysis of variance (ANOVA), Kruskal-Wallis tests, or Fisher-Freeman-Halton tests, where appropriate. All tests used a significance level of P<0.05.
Results
In-hospital deaths
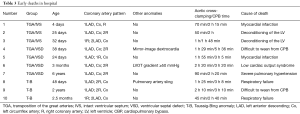
In hospital deaths occurred in 10 (8.4%) patients after ASO. The mortality rate of TGA/IVS, TGA/VSD and Taussig-Bing were 5.1%, 9.3% and 17.6%, respectively (P=0.288). The in-hospital mortality rate decreased significantly over time, from 25.0% (2 of 8) in the first year [2007] to 5.6% (1 of 18) in the final year [2013]. Almost patients died within 24 hours after the operation. The most cause of death was low cardiac output due to deconditioning of the left ventricle and myocardial infarction. Seven patients were applied with a fenestrated patch when VSD repaired and 1 died of severe PH. The mortality rate of patients with abnormal coronary anatomy was higher than those with normal coronary anatomy (20.5% vs. 6.3%, P=0.04). The patient with left ventricular outflow tract obstruction, mirror-image dextrocardia and the one with pulmonary artery sling all died. The detailed information about early deaths in hospital was shown in Table 3.
Full table
Outcomes
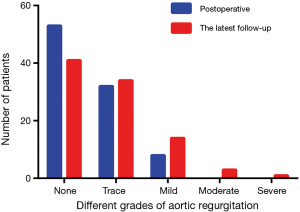
Follow-up was available for 93 (85.3%) patients at a mean duration of 60.7±20.2 months. The follow-up content included valvular function and pulmonary artery stenosis. From the most recent transthoracic echocardiogram, aortic regurgitation was absent in 44.1% (n=41) of patients, trace in 36.6% (n=34) of patients, mild in 15.1% (n=14) of patients, moderate in 3.2% (n=3) of patients and severe in 1.1% (n=1) of patients. Figure 1 showed that neoaortic regurgitation (neoAR) increased progressively over time after the ASO. Tricuspid regurgitation was absent in 40.9% (n=38) of patients, trace in 54.8% (n=51) of patients, mild in 3.2% (n=3) of patients and moderate in 1.1% (n=1) of patients, no patients had severe tricuspid regurgitation. For neopulmonary valve, a total of 61 (65.6%) patients had no regurgitation, 21 (22.6%) patients had trace regurgitation, 7 (7.5%) patients had mild and 4 (4.3%) patients had moderate pulmonary regurgitation, no patients had severe pulmonary regurgitation. A total of 88 (94.6%) patients had no or trivial pulmonary stenosis, 4 (4.3%) had mild stenosis, 1 (1.1%) had moderate stenosis and no patients had severe stenosis (Figure 2). The majority of patients had the highest gradient at the origin of pulmonary branch artery, especially right pulmonary branch artery.
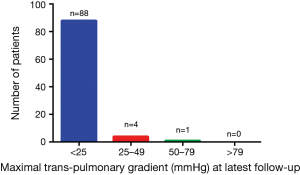
There were 2 (2.2%) patients required a surgical intervention. One patient was diagnosed as TGA/VSD with PH, there was a mild aortic regurgitation after his ASO. Two years later he underwent an interventional closure for residue VSD. The aortic regurgitation progressed to severe and left ventricle enlarged, so he underwent an aortic valve replacement operation 5 years later. The patient recovered well and 3 months later after the second operation, transthoracic echocardiogram showed left ventricle ejection fraction was 60%, left ventricle end-diastolic diameter shrinks from 4.9 to 3.6 cm. The other one was diagnosed as TGA/VSD with coarctation of the aorta, underwent a surgical intervention for residual coarctation of the aorta.
Discussion
Since Jatene performed the first successful ASO in 1975, survival rates have improved with refinement of surgical techniques and improved medical management. It was recently reported that mortality rate has decreased as low as 1.4% (12). However, the ASO remains one of the most complex neonatal operations. In this study, we reviewed early and mid-term results of patients receiving ASO in our department. It included three groups of TGA/IVS, TGA/VSD and Taussig-Bing anomaly, with a median age older than previous reports (13,14). The overall in hospital mortality rate is 8.4%, similar to the mortality rate reported by Rudra et al. (13) and it has decreased to 5.6% in 2013. One cause of death is difficulty with coronary artery transfer which resulting in myocardial ischemia after the operation. Although recent reports suggested that coronary artery pattern was not a predictor of mortality in early postoperative period (15,16), we still thought that unusual coronary artery anomaly (single coronary artery, intramural coronary artery anatomy, etc.) could increase the difficulty of coronary artery transfer and increase the mortality.
For TGA/IVS, the main reason for early mortality is deconditioning of the left ventricle. The upper age limit for a primary ASO for TGA/IVS is 3 weeks. Beyond 3 weeks, left ventricle becomes measurably thinner and left ventricle pressure lower than two-thirds systemic pressure. Consequently, either an atrial switch operation (Senning or Mustard), or a two-stage procedure involving the use of a pulmonary artery band to prepare the left ventricle for a later ASO, should be advocated (17,18). However, late referral of patients with TGA/IVS is common in China. In early periods, we didn’t perform pulmonary artery band procedure for some infants beyond the neonatal period, which led them died of left ventricle failure. It should be avoided in the future.
In infants with TGA plus VSD or TBA, ASO should also be performed as early as possible (preferably within 3 months, but no later than 6 months after birth) to avoid early and rapid development of pulmonary vascular obstructive disease (19,20). For infants brought to the hospital beyond the optimal surgical window, we performed partial closure of VSD with a fenestrated patch, which gave them a chance to a surgery. In our study, most of them recovered well, but for patients with severe PH, it is obviously a great risk for operation and need careful monitoring. Right heart failure will occur slowly although targeted PH therapies were given. In addition, accompanied malformations and pulmonary infection also can increase the mortality rate.
NeoAR is a common complication after ASO. The risk factors for neoAR development are reported to be arterial size discrepancy, previous pulmonary artery band, older age at time of ASO, presence of VSD and the trap-door type of coronary reimplantation (6,21-23). In our study, there was a relatively high incidence of trivial or mild aortic valve regurgitation, but the rate of moderate to severe aortic valve regurgitation was low. Only one patient underwent reoperation due to aortic valve regurgitation. This patient had a moderate VSD and was one and a half years old at the operation, a residue VSD was retained during the ASO because of PH, all of these can be the risk factors for neoAR. Another suspicious factor may be the coronary reimplantation technique. We employed split incision for coronary reimplantation, which will decrease the risk of coronary distortion on the one hand, but enlarge the neoaortic root and increase the risk of aortic regurgitation on the other hand. Lim et al. showed that neoAR was significantly correlated with the size of aortic sinus and aortic sinotubular junction over time (14). Formigari et al. (6) suggested the trap-door type of coronary reimplantation is associated with an increased risk for valvular dysfunction, possibly because of a distortion of the sinotubular junction geometry. For this reason, they recommend the punch technique for repair in all but the most complicated coronary pattern. Our previous studies also suggested that more consideration should be given to sinus diameter and sinotubular junction diameter when we performed aorta reconstruction (24,25). However, it should be mentioned that our surgical reintervention rate for neoaorta wasn’t higher than previous reports (13,22,26), therefore, our technique for coronary reimplantation didn’t influence aortic valve function actually in early and mid-term although we still need long-term follow-up. It is probably because of our control of sinotubular junction diameter. In our study, there was also some incidence of tricuspid regurgitation and pulmonary regurgitation occurred. It is related to the older age at operation for some patients. This part of patients always had PH, which will induce tricuspid and pulmonary regurgitation. The only solution to avoid it is to operate earlier.
Pulmonary artery stenosis is reported to be the most frequent cause for reintervention (27,28). The obstruction was commonly located in the main pulmonary artery or proximal branches, but valvular and subvalvular stenoses were also observed (28). In our study, most patients had the transpulmonary gradient less than 25 mmHg, only one patient had moderate pulmonary stenosis and no patients need reinterventions. This probably related to the surgical methods and material we used for pulmonary artery reconstruction. Classic pulmonary artery reconstruction is generally achieved by filling the defects after coronary ostia explantation with a single inverted bifurcated glutaraldehyde-treated pericardial patch (pantaloon) or with two separate button-shaped pericardial patches. We used two fresh pericardial patches and also extended the length of pulmonary artery. Different with glutaraldehyde-treated pericardial patch, the fresh pericardial patch has the feature of elasticity, therefore, the pulmonary stenosis barely occurred in pulmonary trunk.
In conclusion, our study showed that the in-hospital mortality rate decreased significantly over time, the most causes of death were postoperative myocardial infarction and deconditioning of the left ventricle. The outcomes of the ASO were excellent and the reoperation rate was very low in the early and mid-term follow-up for these relative elder infants.
Acknowledgements
Funding: This study was funded by Hubei Provincial Natural Science Foundation of China (2015CFB466) and grants from the Health and family planning commission of Hubei province (02.01.14039).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- Jatene AD, Fontes VF, Paulista PP, et al. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg 1976;72:364-70. [PubMed]
- Prêtre R, Tamisier D, Bonhoeffer P, et al. Results of the arterial switch operation in neonates with transposed great arteries. Lancet 2001;357:1826-30. [Crossref] [PubMed]
- Prifti E, Crucean A, Bonacchi M, et al. Early and long term outcome of the arterial switch operation for transposition of the great arteries: predictors and functional evaluation. Eur J Cardiothorac Surg 2002;22:864-73. [Crossref] [PubMed]
- Tobler D, Williams WG, Jegatheeswaran A, et al. Cardiac outcomes in young adult survivors of the arterial switch operation for transposition of the great arteries. J Am Coll Cardiol 2010;56:58-64. [Crossref] [PubMed]
- Legendre A, Losay J, Touchot-Koné A, et al. Coronary events after arterial switch operation for transposition of the great arteries. Circulation 2003;108 Suppl 1:II186-90. [Crossref] [PubMed]
- Formigari R, Toscano A, Giardini A, et al. Prevalence and predictors of neoaortic regurgitation after arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg 2003;126:1753-9. [Crossref] [PubMed]
- Moll JJ, Michalak KW, Młudzik K, et al. Long-term outcome of direct neopulmonary artery reconstruction during the arterial switch procedure. Ann Thorac Surg 2012;93:177-84. [Crossref] [PubMed]
- Ullmann MV, Gorenflo M, Bolenz C, et al. Late results after extended pulmonary artery reconstruction in the arterial switch operation. Ann Thorac Surg 2006;81:2259-66. [Crossref] [PubMed]
- Wernovsky G, Sanders SP. Coronary artery anatomy and transposition of the great arteries. Coron Artery Dis 1993;4:148-57. [Crossref] [PubMed]
- Tani LY, Minich LL, Day RW, et al. Doppler evaluation of aortic regurgitation in children. Am J Cardiol 1997;80:927-31. [Crossref] [PubMed]
- Nugent EW, Freedom RM, Nora JJ, et al. Clinical course in pulmonary stenosis. Circulation 1977;56:I38-47. [PubMed]
- Anderson BR, Ciarleglio AJ, Hayes DA, et al. Earlier arterial switch operation improves outcomes and reduces costs for neonates with transposition of the great arteries. J Am Coll Cardiol 2014;63:481-7. [Crossref] [PubMed]
- Rudra HS, Mavroudis C, Backer CL, et al. The arterial switch operation: 25-year experience with 258 patients. Ann Thorac Surg 2011;92:1742-6. [Crossref] [PubMed]
- Lim HG, Kim WH, Lee JR, et al. Long-term results of the arterial switch operation for ventriculo-arterial discordance. Eur J Cardiothorac Surg 2013;43:325-34. [Crossref] [PubMed]
- Fricke TA, Bulstra AE, Naimo PS, et al. Excellent Long-Term Outcomes of the Arterial Switch Operation in Patients With Intramural Coronary Arteries. Ann Thorac Surg 2016;101:725-9. [Crossref] [PubMed]
- Altin FH, Sengul FS, Yildiz O, et al. Impact of Coronary Artery Anatomy in Arterial Switch Procedure on Early Mortality and Morbidity. Congenit Heart Dis 2016;11:115-21. [Crossref] [PubMed]
- Sekarski N, Hurni M, von Segesser LK, et al. Adaptable pulmonary artery band for late arterial switch procedure in transposition of the great arteries. Ann Thorac Surg 2012;94:1311-6. [Crossref] [PubMed]
- Talwar S, Nair VV, Choudhary SK, et al. Atrial switch operation in the current era: modifications and pitfalls. World J Pediatr Congenit Heart Surg 2012;3:96-103. [Crossref] [PubMed]
- Newfeld EA, Paul MM, Muster AJ, et al. Pulmonary vascular disease in complete transposition of the great arteries: a study of 200 patients. Am J Cardiol 1974;34:75-82. [Crossref] [PubMed]
- Clarkson PM, Neutze JM, Wardill JC, et al. The pulmonary vascular bed in patients with complete transposition of the great arteries. Circulation 1976;53:539-43. [Crossref] [PubMed]
- Losay J, Touchot A, Capderou A, et al. Aortic valve regurgitation after arterial switch operation for transposition of the great arteries: incidence, risk factors, and outcome. J Am Coll Cardiol 2006;47:2057-62. [Crossref] [PubMed]
- Hwang HY, Kim WH, Kwak JG, et al. Mid-term follow-up of neoaortic regurgitation after the arterial switch operation for transposition of the great arteries. Eur J Cardiothorac Surg 2006;29:162-7. [Crossref] [PubMed]
- Schwartz ML, Gauvreau K, del Nido P, et al. Long-term predictors of aortic root dilation and aortic regurgitation after arterial switch operation. Circulation 2004;110:II128-32. [Crossref] [PubMed]
- Qiao A, Pan Y, Dong N. Modeling study of aortic root for ross procedure: a structural finite element analysis. J Heart Valve Dis 2014;23:683-7. [PubMed]
- Pan Y, Qiao A, Dong N. Fluid-structure interaction simulation of aortic valve closure with various sinotubular junction and sinus diameters. Ann Biomed Eng 2015;43:1363-9. [Crossref] [PubMed]
- Co-Vu JG, Ginde S, Bartz PJ, et al. Long-term outcomes of the neoaorta after arterial switch operation for transposition of the great arteries. Ann Thorac Surg 2013;95:1654-9. [Crossref] [PubMed]
- Raju V, Burkhart HM, Durham LA 3rd, et al. Reoperation after arterial switch: a 27-year experience. Ann Thorac Surg 2013;95:2105-12; discussion 2112-3. [Crossref] [PubMed]
- Khairy P, Clair M, Fernandes SM, et al. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation 2013;127:331-9. [Crossref] [PubMed]


