Diaphragmatic and pericardial reconstruction after surgery for malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) is an uncommon tumour. More than 80% of cases are associated with occupational exposure to asbestos. The incidence increased over the last 10 years, and this is mainly due to 30–50 years latency from the asbestos exposure to clinical onset of the malignancy.
There are two surgical procedures in the treatment of MPM. According to the IASLC classification: (I) extra-pleural pneumonectomy (EPP) is defined as a complete, en bloc, removal of the whole lung, including the parietal and visceral pleura, the diaphragm and the pericardium; (II) extended pleurectomy/decortication (P/D) is similar but the lung is spared, to obtain a macroscopic resection of the tumour (pulmonary sparing surgery with removal of parietal and visceral pleura) (1).
To date, there is no consensus regarding the best management of patients affected by MPM, and in most centres worldwide generally surgery is offered as part of a multi-modality treatment. EPP is associated with a higher rate of peri-operative and postoperative morbidity and mortality due to the pneumonectomy step, whereas in P/D the most frequent complication is represented by prolonged air-leak and the risk of bleeding.
The diaphragmatic and pericardial reconstructions are in any case mandatory to avoid gastric and cardiac herniation: they still are associated with the risk of dehiscence or technical failure due to the positive abdominal pressure and—especially on the left side in the costo-phrenic corner, near the oesophageal hiatus—to the lack of diaphragmatic tissue which could be adopted to anchor the prosthesis.
The present manuscript is focused on technical issues related to the step of the reconstruction of both hemidiaphragm and pericardium and associated complications; only a summary is included regarding the timing of resection.
Reconstruction of the diaphragm
It is usually performed through a traditional posterolateral thoracotomy in the fifth intercostal space; latissimus and serratus anterior muscles are divided. The incision begins 3 to 5 cm from the costo-vertebral angle posteriorly, and it extends anteriorly to the costochondral junction. After incising the lateral margin, the diaphragmatic attachments are avulsed, and the peritoneum is dissected off the inferior surface of the diaphragm by blunt dissection.
Prosthetic materials
Autologous or alloplastic materials can be used for the reconstructive step of the procedure. The market offers many types of prosthetic products made of different material. There is no robust evidence which guides the choice, and this is mainly left to the surgeon's preference. Within the autologous options, we encounter the reconstructions with pedicled muscle flaps: external oblique flap, latissimus dorsi reverse flap and TRAM. Bedini et al. (2) described the technique which was more commonly adopted worldwide. The group employed the latissimus dorsi during surgery for mesothelioma or for resection of large sarcomas which involved the diaphragm. They described interesting results.
The pros of the autologous options are: reduced risk of infection related to the prosthetic material, and no permanent foreign bodies are left in situ but vascularised tissue. The cons are all the potential complications related to the harvesting procedure (donor-site complication, graft failure, arm impaired mobility, etc.). Also, the overall procedure and anaesthesia time are more extended because of the harvest, which is time-consuming.
Recently, with increased development of safe prosthetic options, the reconstruction is more often accomplished by meshes. These are either bio-prosthetic, synthetic materials either entirely artificial, absorbable, or non-absorbable.
Expanded polytetrafluoroethylene (PTFE, Trademark Gore-Tex), 2-mm thick, is the most recommended material for reconstruction of the diaphragm following EPP or P/D. Studies have demonstrated fibroblastic invasion and epithelial growth over the mesh after 4 months, and the foreign body is fully integrated with the host after seven months. PTFE is an inert polymer of monofilament threads which provides a firm but soft texture. It has adequate sealant properties but is not elastic, tends to shrink and produce more scar tissue than other materials. Other options include the composite variant Gore Dual mesh, material that has a double surface, that minimise the adhesions with the abdominal organs while favour the host cellular in-growth, the Mersilene mesh, the Prolene polypropylene mesh (both from Ethicon, Somerville, US) and the Marlex polypropylene monofilament mesh (Davol, Cranston, RI, USA) which is more rarely adopted.
Although less experience is available, biologic materials are newer alternative tools for the reconstruction of diaphragmatic defects: acellular porcine collagen (Permacol™, Covidien, AG, USA), acellular human dermis (AlloDerm™, LifeCell Corporation, Branchburg, NJ, USA), bovine pericardium and, finally, the composite mesh, polypropylene plus AlloDerm, with the least facing the abdomen. Biologic meshes are produced with the adoption of advanced technologies which eliminate the cells and so the immunogenic properties of the allograft. The framework of the basal membrane is re-colonised by the host epithelium. The extracellular matrix will be degraded, and the host will produce novel collagen until the full incorporation of the graft. While this process is completed the animal material ensure the integrity of the reconstruction and provide strength.
In 2017, Rolli et al. published encouraging results with the adoption of Surgimesh-Pet (Aspide Medical, FR). This is a synthetic mesh made of multifilament polyester which creates a 3-D texture. This is inert, strong, non-absorbable material but permeable to cells and fluids migration. It provides a reliable framework for the incorporation (3).
Technique of reconstruction
The patch can be sutured in place using interrupted, zero, non-absorbable sutures, starting from the most medial aspect. At this level it is stitched to the inferior margin of pericardium or the pericardial prosthesis (in cases of concurrent pericardial resection); anteriorly and laterally the diaphragmatic mesh is anchored around the ribs and lastly to the posterior chest wall (Figure 1). Gore-Tex suture can be passed through the chest wall using an awl and then tied over a small piece of PTFE, to prevent the sutures from pulling through the patch.
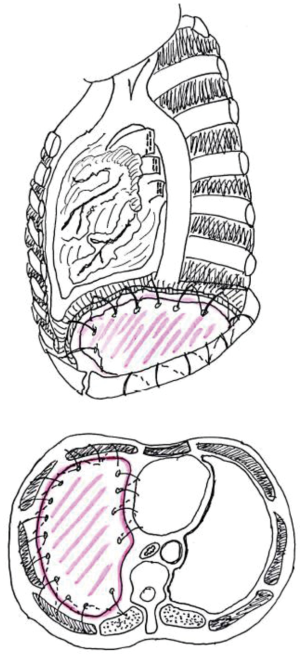
On the lateral and posterior aspect, it is pivotal to anchor the prosthesis to the chest wall (with sutures going around the ribs) and to the diaphragmatic crus. The neo-diaphragm can then be tacked to the oesophagus using finer sutures (3-0 Prolene).
The prosthesis should be placed at the same level of the typical anatomic position of the muscle, such as at the level of the 10th interspace posteriorly and the 8th/9th space on the anterior and lateral aspect. This is essential, according to some authors, to prevent collateral damage to the abdominal viscera when the patient is undergoing adjuvant radiation treatment (oesophagus and stomach on the left, liver on the right). The adoption of intensity modulated radiation therapy (IMRT) minimises these risks since allows precise delivery of the radiations.
The personal experience of the authors suggests that there is a tendency to place the mesh lower, and with steeper angle than the native muscle. Therefore, we recommend to suture in the appropriate intercostal spaces and accept a result which will give a slightly elevated diaphragm when compared with preoperative findings. This prevents to stitch in unsafe locations, and the procedure results technically easier. The most common site of dehiscence is the left posteromedial costo-phrenic recess, with the incidence of herniation of up to 7%. In fact, the anatomy of this region makes the repair more challenging. The presence of oesophageal and aortic hiatus does not offer an available tissue to anchor the prosthesis. Sugarbaker et al. described their technique to reduce the risk of dehiscence at this fine site. They suggested leaving a 2-cm rim of the native diaphragm, surrounding the two hiati, which provides enough tissue to anchor the prosthesis and rule out a weak point in the novel diaphragm (Figure 2) (4). The authors prefer to place an L-shaped titanium plate, screwed in the 9th and 10th vertebral bodies and, also, in the corresponding rib’s posterior arch. The vertebral bodies and the posterior rib arch need to be appropriately exposed before plate fixation. To expose the periosteum and identify the site for the screw is a crucial step as it prevents to screw on the intervertebral disk.
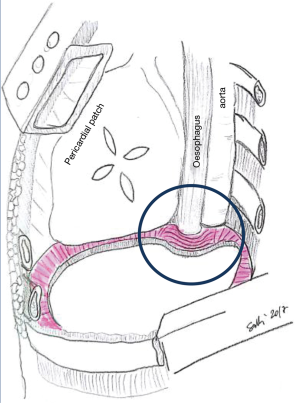
Beforehand, a simulation, accommodating a bending, L-shaped, plate model, should be carried out. This plate should adhere to the two vertebral bodies and the rib’s posterior arch as shown in Figure 3.
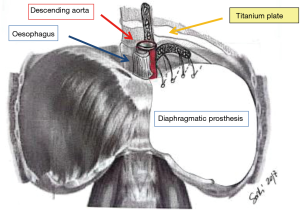
The bone of the vertebral bodies is then exposed, removing all the parietal pleura and the endothoracic fascia, so that the plate is secured with two screws (14 mm) on the two consecutive vertebral bodies. One or two screws (12 mm) go to the corresponding rib. The patch is now fixed to the plate with interrupted sutures, which is passed through the plate’s oval openings. The plating adds 15 minutes to the entire operation but provides reliable support to anchor the prosthesis. The above technique was described in 2010 by Schiavon et al. (5).
Also on the right side, and for apparent reason, the reconstruction is more challenging on the mediastinal side. A notch in the mesh must be suited around the inferior vena cava to avoid inferior vena cava and hepatic veins stenosis. However, the presence of the liver makes the risk of abdominal viscera’s herniation much less likely. Therefore, even if the reconstruction is left incomplete on the medial side, this will be safe, and the chances of herniation are meagre.
Tips and pitfalls
- Create a dynamic patch repair reduce the risk of dehiscence;
- The reconstruction should not over-correct the natural position of the native diaphragm and all (!) the anchoring points must be as safe as possible;
- Because of the anatomy of the left posteromedial costo-phrenic recess care should be taken to avoid iatrogenic hernias;
- For right-sided procedures, the hepatic veins and the inferior vena cava must be kept in mind during resection and reconstruction: it is essential to avoid stenosis of the inferior vena cava and hepatic veins.
Pericardial reconstruction
The site and the extent of the pericardiectomy are both critical. The primary goal is to rule out cardiac herniation. After a right-sided procedure, the torsion of the heart over the superior and inferior vena cava produces grave hemodynamic consequences. The hemodynamic injury is fewer on the left side, but cardiac arrest can result from strangulation of the left ventricle. Ischemic damage to the myocardium could result. Therefore, consequent conduction disorders (e.g., ventricular fibrillation) could happen. Therefore, the golden rule is to always reconstruct the pericardial defect on both sides.
Choice of prosthetic material
Again as for diaphragmatic defects, pericardial defects during surgery for MPM are usually replaced by several types of materials, either artificial or biologic.
It is preferable to accomplish the pericardioplasty by replacement with synthetic mesh. All or most of the defect should be covered by the prosthesis, which can be absorbable or not. The least ones are divided into impermeable and permeable. Impermeable is the PTFE (4,6-8) (Gore-Tex, WL Gore & Associates, Phoenix, USA) while in the second group we count: prolene double-filamented polypropylene mesh (Ethicon Inc., Somerville, USA), Marlex polypropylene monofilament fibre mesh (Davol Inc., Cranston, RI, USA) Mersilene multifilament polyester fibre mesh (Ethicon, Inc., Somerville, USA) etc. The permeable one should reduce the chance of postoperative tamponade.
Constrictive pericarditis has been associated with the use of Marlex for the reconstruction of the pericardium after cardiac surgery (9). The risk of infection is, in general, one of the weaknesses of the non-absorbable prosthesis.
Absorbable materials such as polyglactin (Vicryl) or polyglycolic acid (Dexon) have also experimented, but there are unsolved issues concerning their strength and their time to absorption. The median absorption time is four months. This should be enough to allow the formation of fibrous cardio-pericardial adhesions which permanently stabilise the heart. Although we know that cardiac herniation occurs more often during or within the first 24 hours after surgery, also cases of late herniations, 6 months afterwards, were reported (10). To date, pericardioplasty with synthetic meshes (for example 15×20 cm of 0.1 mm thin Gore-Tex Pericardium Membrane, WL Gore and Associates, Inc.) is the preferred method of pericardial reconstruction.
Technique of reconstruction
A too tight pericardial patch can result in cardiac tamponade as the ventricle is constricted and the diastolic function is impaired. To prevent these complications, the patch can be secured in place using interrupted 0 non-absorbable sutures several types of sutures can be utilised, starting from the posterior pericardium, which is deeper thus facilitating the path sizing when suturing it to the anterior pericardium.
An inadequately fenestrated pericardial patch may cause cardiac tamponade because of the build-up of serum under the patch.
The steps of the reconstruction are as follows (11-13):
- Assessment of the size of the defect and choice of the replacing material;
- Multiple interrupted stitches are performed 1 to 2 cm apart through the margin of the remaining pericardium, starting from the more in-depth aspect (running sutures are not ideal in this context because increase the risk of dehiscence);
- The patch is tailored to cover the defect and secured with the already placed sutures; few additional stitches are occasionally needed for securing the mesh and to re-establish a shape like the normal anatomy.
Care must be taken to avoid constriction during the pericardioplasty, especially on the right side: in fact, it should always recall the modifications occurring into the pneumonectomy space in the long-term period, with a significant mediastinal shift and usually with the increase of the cardiac chamber volume. The golden rule says that, after reconstruction, two/three fingers should quickly pass through the superior hole of the prosthesis.
Tips and pitfalls
- Left pericardial defects after EPP or P/D should always be reconstructed;
- The pericardium must be reconstructed in a geometric, tri-dimensional and tension-free (!) fashion to avoid constriction;
- Impermeable prosthesis could be fenestrated as post-operative tamponade prophylaxis;
- The mesh should be anchored, with interrupted sutures, to the margins of the remaining pericardium only and avoid the surrounding tissues.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol 2011;6:1304-12. [Crossref] [PubMed]
- Bedini AV, Andreani SM, Muscolino G. Latissimus dorsi reverse flap to substitute the diaphragm after extrapleural pneumonectomy. Ann Thorac Surg 2000;69:986-8. [Crossref] [PubMed]
- Rolli L, Leuzzi G, Girotti P, et al. Permeable Nonabsorbable Mesh for Total Diaphragmatic Replacement in Extended Pneumonectomy. Ann Thorac Surg 2017;104:e105-e107. [Crossref] [PubMed]
- Sugarbaker DJ, Jaklitsch MT, Bueno R, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2004;128:138-46. [Crossref] [PubMed]
- Schiavon M, De Filippis A, Marulli G, et al. A new technique of diaphragmatic patch fixation in extrapleural pneumonectomy. Eur J Cardiothorac Surg 2010;38:798-800. [Crossref] [PubMed]
- Deiraniya AK. Cardiac herniation following intrapericardial pneumonectomy. Thorax 1974;29:545-52. [Crossref] [PubMed]
- Shimizu J, Ishida Y, Hirano Y, et al. Cardiac herniation following intrapericardial pneumonectomy with partial pericardiectomy for advanced lung cancer. Ann Thorac Cardiovasc Surg 2003;9:68-72. [PubMed]
- Veronesi G, Spaggiari L, Solli PG, et al. Cardiac dislocation after extended pneumonectomy with pericardioplasty. Eur J Cardiothorac Surg 2001;19:89-91. [Crossref] [PubMed]
- Chen RF, Lai CP. Constrictive pericarditis associated with Marlex mesh. Two case reports. Tex Heart Inst J 2001;28:63-4. [PubMed]
- Urschel JD, Takita H. Pericardial closure after intrapericardial pneumonectomy. Ann Thorac Surg 1999;67:295-6. [PubMed]
- Goldstraw P, Jiao X. Pericardial repair after extensive resection: another use for the pedicled diaphragmatic flap. Ann Thorac Surg 1996;61:1112-4. [Crossref] [PubMed]
- DaSilva MC, Sugarbaker DJ. Technique of Extrapleural Pneumonectomy. Oper Tech Thorac Cardiovasc Surg 2010;15:282-93. [Crossref]
- Finley DJ, Abu-Rustum NR, Chi DS, et al. Reconstructive techniques after diaphragm resection. Thorac Surg Clin 2009;19:531-5. [Crossref] [PubMed]


