Serum osteopontin in patients with lung cancer and chronic obstructive pulmonary disease: does the co-existence make the difference?
Introduction
Chronic obstructive pulmonary disease (COPD) and lung cancer (LC) are causally related to cigarette smoking and there is increasing evidence linking the two diseases beyond a common etiology. COPD is an independent risk factor for LC and LC is up to five times more likely to occur in smokers with airflow obstruction than those with normal lung function. The high prevalence of LC in COPD patients suggests that these two entities may share similar mechanisms (1). Nevertheless, a causal relationship between COPD and lung tumorigenesis is not yet known. It is generally accepted that chronic inflammation plays a central role in the pathogenesis of COPD and lung tumorigenesis. Interestingly, patients with COPD treated with high-dose inhaled corticosteroids present reduced incidence of LC and death, suggesting that inhibition of inflammation can halt lung tumorigenicity (2).
In an effort to detect LC at an earlier stage, non-invasive biomarkers could potentially enhance diagnostic management by minimizing the use of costly tests such as PET-CT and lowering radiation exposure associated with CT monitoring (3).
Osteopontin (OPN) is a matricellular protein involved in tissue remodeling, cell-mediated immunity and malignant transformation. OPN, that is produced by osteoclasts, macrophages, T cells, kidney cells, and vascular smooth muscle cells, regulates normal physiological processes, including bone resorption, wound healing, tissue remodeling, immunological responses and vascularization, as well as various pathophysiological conditions (4).
OPN has been involved in tumor progression and angiogenesis and is over expressed in LC. Immunohistochemical staining in non-small cell lung cancer (NSCLC) patients who underwent surgical resection showed that OPN expression was significantly associated with gender, TNM stage and tumor differentiation. In this study it was suggested that tumor stage, lymph-node metastases and OPN expression were independent prognostic factors for NSCLC (5). OPN has been evaluated as a biomarker of disease progression and has been correlated with poor survival (6). OPN levels were higher in sputum of patients with COPD compared with healthy subjects and a possible role of this cytokine in neutrophilic inflammation and in the pathogenesis of emphysema was suggested (7). However, the role of OPN in the pathogenesis of both COPD and LC has not been fully elucidated.
The aim of the present study was to determine the effect of the co-existence of LC (LC) and COPD in serum OPN levels and to estimate OPN prognostic performance in 1-year mortality in LC and its diagnostic value for LC in pre-existing COPD.
Methods
Patients
All subjects were recruited from the 1st and 2nd Respiratory Medicine University Departments in Athens between April 2011 and February 2014. All COPD patients had a diagnosis confirmed by a respiratory physician, prior to LC occurrence. Diagnosis and staging of COPD were established according to GOLD guidelines (8). Forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were measured using Master Screen Body (Viasys Healthcare, Jaeger, Hoechberg, Germany) according to the American Thoracic Society guidelines (9).
Diagnosis of LC was confirmed by pathology, including bronchoscopic biopsy, percutaneous lung biopsy, metastatic lymph node biopsy and pleural fluid cytology. LC staging was determined using the updated 2009 International Association for the Study of Lung Cancer (IASLC) 7th tumor node metastasis (TNM) classification (10).
All LC and COPD patients were current or ex-smokers. Patients with a history of respiratory disease other than COPD or any concomitant malignant (other than LC), heart, renal, liver or collagen disease were excluded. Patients with a respiratory tract infection or COPD exacerbation in the past 8 weeks prior to admission, as well as those with low performance status (PS:IV), inability or unwillingness to collaborate with the investigators were also excluded. All patients received optimal treatment for LC and COPD according to their attending physicians and were followed-up for at least 1 year or until death. The study protocol was approved by the local ethic committees of both hospitals and all participants provided an informed consent.
OPN measurement
Blood samples were collected at the time of diagnosis before the initiation of any kind of treatment for LC. The samples were centrifuged at 1,500 ×g for 10 min and serum was collected and stored at −80 °C until analysis. Serum OPN levels were measured using commercially available enzyme-linked immunosorbent assay kits with specific monoclonal antibodies against OPN (R&D Systems, Minneapolis, MN, USA) with a cut-off value OPN:MDD (median detective density) of 0.22 ng/mL.
Study outcomes
The main outcome of the study was to explore the effect of the co-existence of LC and COPD on serum OPN levels vs. COPD and LC. Secondary outcomes included prognostic performance of OPN in 1-year mortality in LC and diagnostic performance of OPN for LC in patients with COPD. Furthermore, possible differences in serum OPN levels according to the histological type of LC [NSCLC vs. small cell lung cancer (SCLC)] or/and the extent (TNM stage for NSCLC and limited/extended for SCLC) of the disease were assessed.
Statistical analysis
Normally distributed data are presented as mean ± standard deviation (SD), skewed data as median (interquartile ranges) and categorical data as n (%). Normality of distribution was checked with Kolmogorov-Smirnov test. Differences in numerical variables between two groups were evaluated with unpaired t-tests or Mann-Whitney U-tests for normally and skewed data respectively, whereas comparisons of proportions were performed using chi-square tests. Statistical comparisons for more than two groups were performed with Kruskal-Wallis test (skewed data) accompanied by an appropriate post-hoc test for multiple comparisons (Dunn’s). For the assessment of OPN as predictor of 1-year mortality and its diagnostic performance for LC among COPD patients, a receiver operating characteristic (ROC) curve was created. Area under the ROC curve (AUC) with 95% confidence intervals (CI) and their differences from 0.5 was calculated. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for the optimal cut-off value.
Using the cut-off value from the ROC curve we dichotomized the study group. A univariate Cox regression analysis and then a multivariate one were performed in order to evaluate the performance of OPN in 1-year mortality. As possible confounders in the above model age, gender, presence or absence of COPD, FEV1% predicted, FEV1/FVC ratio, histological type, body mass index (BMI), current or ex smoking, smoking in pack-years, and extent of the disease were entered. Results were presented with hazard ratios (HR) with 95% confidence intervals (CI). The 1-year survival according to the cut-off value for OPN was evaluated with a Kaplan-Meier survival curve and the respective log-rank test. Statistical analysis was performed with SPSS 16.0 (Chicago, IL, USA), Graph Pad Prism 5 (Graph Pad Software Inc, La Jolla, CA, USA) and MedCalc 9 (MedCalcSoft ware, Mariakerke, Belgium).
Results
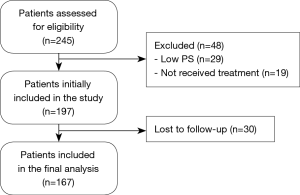
The flow chart of the study participants is shown in Figure 1.
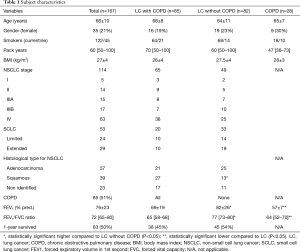
Demographic characteristics of the study group are presented in Table 1.
Full table
Serum OPN levels in LC and/or COPD
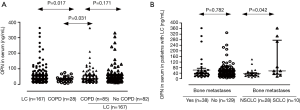
OPN levels were significantly higher in patients with LC compared to those with COPD alone [median 40 (IQR, 24–63) vs. 32 (IQR, 19–36.5) ng/mL, P=0.017, Figure 2A]. OPN levels were significantly higher in patients with COPD and LC compared to those with COPD alone [median, 36 (IQR, 23–59) vs. 32 (IQR, 19.0–36.5) ng/mL, P=0.031]. No significant difference was observed between patients with LC and COPD vs. those with LC without COPD [median, 36 (IQR, 23–59) vs. 45 (IQR, 24–69) ng/mL, P=0.171, Figure 2A].
Serum OPN levels in histological subtypes of LC and stage of the disease
OPN serum levels did not significantly differ according to the histological type [median, 42 (IQR, 26–64) vs. 39 (IQR, 24–63) ng/mL for SCLC and NSCLC respectively, P=0.565]. For SCLC, no significant difference in serum OPN levels (ng/mL) was found between patients with extensive and those with limited disease [median IQR 38 (IQR, 21–74) vs. 53 (IQR, 27–63) ng/mL respectively, P=0.761]. We also divided NSCLC patients in three different subgroups according to staging. Stage I & II comprised one group, stage III another and stage IV was the third group. OPN serum levels did not significantly differ among the three NSCLC subgroups [median, 30 (IQR, 19–39) ng/mL for stages I & II, 45 (IQR, 26–85) ng/mL for stages IIIA & IIIB, and 40 (IQR, 25–63) ng/mL for stage IV, P=0.091]. Serum OPN levels did not differ between patients with or without bone metastasis, P=0.782] (Figure 2B). However, when patients with bone metastasis were stratified according to histological type, those with SCLC presented with significantly higher serum OPN levels compared to NSCLC [median, 56 (IQR, 53–276) vs. 36 (IQR, 23–43) ng/mL for SCLC and NSCLC respectively, P=0.042] (Figure 2B).
Serum OPN levels and 1-year mortality
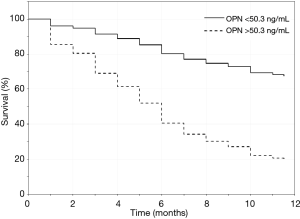
The prognostic performance of serum OPN (cut-off value ≥50.3 ng/mL) for the identification of 1-year mortality in patients with LC was assessed by ROC curve analysis which showed an AUC 0.84 (P<0.001). Sensitivity was 90% while specificity was 64% with PPV 72% and NPV 86% (Figure 3). Patients were divided into two groups according to serum OPN levels.

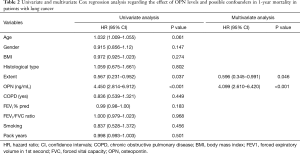
Univariate and multivariate Cox regression analysis regarding the prognostic effect of serum OPN levels in 1-year mortality are presented in Table 2. In the univariate Cox regression analysis, OPN levels ≥50.3 ng/mL and disease extent were associated with higher risk of 1-year mortality, with OPN being the stronger predictor. In the multivariate analysis, the higher OPN levels remained an independent predictor of 1-year mortality while extent of the disease showed a weak significance.
Full table
Kaplan-Meier curves evaluating 1-year survival for LC patients are presented in Figure 4. LC patients with serum OPN levels ≥50.3 ng/mL displayed worse 1-year survival compared to those with serum OPN levels <50.3 ng/mL (P<0.001 the comparison by log-rank test).
Performance of serum OPN levels for the diagnosis of LC in patients with COPD
The diagnostic value of serum OPN levels for the identification of concomitant LC in patients with COPD was evaluated by performing a ROC curve analysis. Serum OPN levels could predict the presence of LC in patients with COPD with a cut-off level of 35 ng/mL (AUC =0.636, 95% CI: 0.540–0.725; sensitivity: 56.5%; specificity: 75%; PPV: 87.3%; NPV: 36.2%).
Discussion
Our study showed that serum OPN levels are higher in patients with LC compared to those with COPD. We also found that serum OPN levels are higher in patients with concomitant LC and COPD compared to those with COPD alone, while in patients with LC the co-existence of COPD did not seem to influence serum OPN levels. Furthermore, in LC patients the higher serum OPN levels were found irrespective of the underlying histological subtype and the extent of the disease. Bone metastases seem to affect the above levels only in patients with SCLC. Finally, using a specific cut-off value as derived from ROC analysis, serum OPN had a good prognostic performance in regard to 1-year survival in LC patients.
Both LC and COPD represent a huge, worldwide, preventable disease burden owing mainly to cigarette smoking (8,11). These diseases occur as co-morbidities at a higher rate than if they were independently triggered by smoking (12). Therefore, it is ultimate to find sensitive parameters to detect early lung alterations associated with chronic tobacco smoke exposure. Exhaled breath condensate (EBC) and electronic nose are non-invasive techniques and the measurement of biomolecules could possibly prove suitable for early identification of healthy smokers at higher risk for tobacco-induced lung damage (13,14). OPN is up regulated in the airways of patients with COPD and is related to inflammatory molecules, such as metalloproteinases (MMPs) and IL-8 which have been implicated in LC pathogenesis and prognosis (15). In a previous study including COPD patients, sputum OPN was closely related to the presence of emphysema as assessed by a HRCT scoring system (7,16). Conflicting results exist in regard to the association between emphysema on CT scan and LC. In a high-risk population, emphysema was independently related to LC (17,18). In our study, we have observed that in LC patients COPD did not further increase OPN levels, while COPD patients with LC expressed higher serum OPN levels compared to patients with COPD alone. Our findings indicate that LC might be the main factor associated with the increase of serum OPN levels.
Contradictory data exist on studies regarding OPN levels and LC stage. Advanced stages of NSCLC had higher values of OPN compared to the early ones (19). Another study showed no significant difference in regard to TNM classification and OPN levels (20). Similar results were observed in our study where SCLC patients were also included. The absence of statistical significance may be attributed to the somehow limited number of patients with early stage disease, which to our knowledge is more compatible to what we see in every day clinical practice.
Conflicting results exist with regard to bone metastasis and OPN levels. NSCLC patients with bone metastasis had an increase in receptor activator of nuclear factor κB ligand, OPN and osteoprotegerin (21). Experimental data showed that OPN-deficient mice had an increased risk for bone metastasis (22). Although OPN protein expression in surgical resected NSCLC failed to predict the future development of bone metastasis (23), OPN levels did not significantly differ between patients with and without bone metastasis (19). In another study, Karapanagiotou et al. (24) showed that OPN levels were significantly higher in patients with NSCLC and bone metastasis. However, this study consisted of a small number of patients and did not examine the effect of OPN in bone metastasis in SCLC. We did not find a difference in OPN levels between LC patients with or without bone metastasis, but we found that SCLC patients with bone involvement had higher OPN levels compared to those with NSCLC and bone involvement. This has never been observed in previous studies and might be attributed to the low number of SCLC with bone metastasis or alternatively might indicate a more intense up regulation of OPN among SCLC patients. Furthermore we did not find a difference in serum OPN levels between squamous cell and adenocarcinoma. This is in contrast to the findings of another study showing that OPN levels of squamous cell carcinoma were significantly higher than those of adenocarcinoma (25).
High serum OPN levels have been shown to predict poor prognosis in NSCLC. Rud et al. (20) showed that a median serum OPN level of 32.9 ng/mL correlated with poor prognosis in NSCLC. In the same study, subgroup analysis of patients with stage I–II disease alone showed that OPN expression was significantly associated with both relapse-free and overall survival, indicating that OPN might be a promising biomarker in early stage NSCLC. Accordingly, it was suggested that OPN should be further explored in the selection of patients for adjuvant treatment following surgical resection. In another study, low serum OPN levels were significantly associated with a favorable prognosis in advanced NSCLC (26). Two recent meta-analyses confirmed that OPN seems to be a good prognostic biomarker of the overall survival in NSCLC patients (27,28). These findings are in accordance with the satisfactory prognostic performance of OPN in 1-year survival observed in our study.
Serum OPN could moderately predict the presence of LC among patients with pre-existing COPD, although this finding was observed in a relatively small number of COPD patients.
Our study has some limitations. First, the number of patients with early-stage NSCLC was small. However, LC does not frequently give early symptoms and therefore it is usually diagnosed at a later stage. Secondly, although smoking was included in the regression analysis, we cannot exclude that current smoking may affect OPN levels since it has been reported that sputum OPN levels were elevated in patients with COPD (7), smoking-related interstitial lung disease (29) and smoking-induced asthma (30). Although our patients were all current or ex-smokers it should be noted that both LC and COPD may occur among non-smokers and this has not been evaluated in this study. A selection of a larger number of patients with a particular histological type and a unanimous current smoking status may help to clarify better the possible effects on serum OPN levels. Thirdly, we did not assess OPN expression immunohistochemically, which might better reflect the role of OPN compared to its serum levels. Finally, the number of patients with COPD alone was rather small. A larger number may compensate for the relative low sensitivity and specificity values and further strengthen the possible diagnostic performance of OPN for LC among COPD patients. Finally, a power analysis to calculate a formal sample size was not performed. However, this is an exploratory study that aimed to explore the effect of the co-existence of LC and COPD on serum OPN levels vs. COPD and LC alone. We have therefore used all consecutive available patients from our outpatient clinic. However, the proper characterization of the study participants has allowed us to perform an appropriate interpretation of the results. The validation of our findings in different cohorts with larger number of patients is required in order to confirm their significance and applicability.
Conclusions
COPD did not affect serum OPN levels in patients with LC. In patients with LC and/or COPD, LC seems to be the major determinant for serum OPN levels. Although not related to the extent of LC, serum OPN seems to be able to predict 1-year mortality in patients with LC. Accordingly, serum OPN might be a promising prognostic biomarker for the survival of LC patients and possibly a diagnostic biomarker of LC among COPD patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This prospective study was performed after having been approved by the ethic committees of both hospitals (Sotiria Chest Hospital no 34889, and Attikon University Hospital, no 754). All participants provided an informed consent.
References
- Durham AL, Adcock I. The relationship between COPD and lung cancer. Lung Cancer 2015;90:121-7. [Crossref] [PubMed]
- Parimon T, Chien JW, Bryson CL, et al. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:712-9. [Crossref] [PubMed]
- Birse CE, Lagier RJ, FitzHugh W, et al. Blood-based lung cancer biomarkers identified through proteomic discovery in cancer tissues, cell lines and conditioned medium. Clin Proteomics 2015;12:18. [Crossref] [PubMed]
- Han S-S, Lee S-J, Kim WJ, et al. Plasma osteopontin is a useful diagnostic biomarker for advanced non-small cell lung cancer. Tuberc Respir Dis (Seoul) 2013;75:104-10. [Crossref] [PubMed]
- Lin Q, Guo L, Lin G, et al. Clinical and prognostic significance of OPN and VEGF expression in patients with non-small-cell lung cancer. Cancer Epidemiol 2015;39:539-44. [Crossref] [PubMed]
- Yan CH, Lv M, Li H, et al. Osteopontin is a novel prognostic biomarker in early-stage non-small cell lung cancer after surgical resection. J Cancer Res Clin Oncol 2015;141:1371-8. [Crossref] [PubMed]
- Papaporfyriou A, Loukides S, Kostikas K, et al. Increased levels of osteopontin in sputum supernatant in patients with COPD. Chest 2014;146:951-8. [Crossref] [PubMed]
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532-55. [Crossref] [PubMed]
- Standardization of Spirometry. 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995;152:1107-36. [Crossref] [PubMed]
- Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:694-705. [Crossref] [PubMed]
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [Crossref] [PubMed]
- Barnes PJ, Celli B. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009;33:1165-85. [Crossref] [PubMed]
- Santini G, Mores N, Penas A, et al. Electronic Nose and Exhaled Breath NMR-based Metabolomics Applications in Airways Disease. Curr Top Med Chem 2016;16:1610-30. [Crossref] [PubMed]
- Malerba M, Montuschi P. Non-Invasive Biomarkers of Lung Inflammation in Smoking Subjects. Curr Med Chem 2012;19:187-96. [Crossref] [PubMed]
- Davies KJ. The complex interaction of matrix metalloproteinases in the migration of cancer cells through breast tissue stroma. Int J Breast Cancer 2014;2014:839094. [Crossref] [PubMed]
- Park KJ, Bergin CJ, Clausen JL. Quantitation of Emphysema with Three-dimensional CT Densitometry: Comparison with Two-dimensional Analysis, Visual Emphysema Scores, and Pulmonary Function Test Results 1. Radiology 1999;211:541-7. [Crossref] [PubMed]
- Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008;178:738-44. [Crossref] [PubMed]
- de-Torres JP, Wilson DO, Sanchez-Salcedo P, et al. Lung cancer in patients with chronic obstructive pulmonary disease. Development and validation of the COPD Lung Cancer Screening Score. Am J Respir Crit Care Med 2015;191:285-91. [Crossref] [PubMed]
- Chang YS, Kim HJ, Chang J, et al. Elevated circulating level of osteopontin is associated with advanced disease state of non-small cell lung cancer. Lung Cancer 2007;57:373-80. [Crossref] [PubMed]
- Rud AK, Boye K, Øijordsbakken M, et al. Osteopontin is a prognostic biomarker in non-small cell lung cancer. BMC Cancer 2013;13:540. [Crossref] [PubMed]
- Terpos E, Kiagia M, Karapanagiotou EM, et al. The clinical significance of serum markers of bone turnover in NSCLC patients: surveillance, management and prognostic implications. Anticancer Res 2009;29:1651-7. [PubMed]
- Nemoto H, Rittling SR, Yoshitake H, et al. Osteopontin deficiency reduces experimental tumor cell metastasis to bone and soft tissues. J Bone Miner Res 2001;16:652-9. [Crossref] [PubMed]
- Zhang L, Hou X, Lu S, et al. Predictive significance of bone sialoprotein and osteopontin for bone metastases in resected Chinese non-small-cell lung cancer patients: a large cohort retrospective study. Lung Cancer 2010;67:114-9. [Crossref] [PubMed]
- Karapanagiotou EM, Terpos E, Dilana KD, et al. Serum bone turnover markers may be involved in the metastatic potential of lung cancer patients. Med Oncol 2010;27:332-8. [Crossref] [PubMed]
- Frey AB, Wali A, Pass H, et al. Osteopontin is linked to p65 and MMP-9 expression in pulmonary adenocarcinoma but not in malignant pleural mesothelioma. Histopathology 2007;50:720-6. [Crossref] [PubMed]
- Isa S, Kawaguchi T, Teramukai S, et al. Serum Osteopontin Levels are Highly Prognostic for Survival in Advanced Non-small Cell Lung Cancer: Results from JMTO LC 0004. J Thorac Oncol 2009;4:1104-10. [Crossref] [PubMed]
- Wang Y, Yang J, Liu H, et al. The association between osteopontin and survival in non-small-cell lung cancer patients: a meta-analysis of 13 cohorts. Onco Targets Ther 2015;8:3513. [PubMed]
- Zou XL, Wang C, Liu K, et al. Prognostic significance of osteopontin expression in non-small-cell lung cancer: A meta-analysis. Molecular and clinical oncology 2015;3:633-8. [Crossref] [PubMed]
- Prasse A, Stahl M, Schulz G, et al. Essential role of osteopontin in smoking-related interstitial lung diseases. Am J Pathol 2009;174:1683-91. [Crossref] [PubMed]
- Hillas G, Loukides S, Kostikas K, et al. Increased levels of osteopontin in sputum supernatant of smoking asthmatics. Cytokine 2013;61:251-5. [Crossref] [PubMed]