Ventilator-associated events after cardiac surgery: evidence from 1,709 patients
Introduction
Cardiac surgery, especially cardiopulmonary bypass (CPB), easily triggers abnormal inflammatory responses, leading to impaired lung function (1). Hence, patients who have undergone heart operation are at high risk for nosocomial pulmonary infection. For decades, ventilator-associated pneumonia (VAP) surveillance has traditionally been used to measure the quality care for mechanically ventilated patients (2). Once postoperative patients are infected with VAP in intensive care unit (ICU), poor prognosis will definitely occur. According to the results of our previously published meta-analysis, the prevalence of VAP is up to 6.37% in this subpopulation, and it is closely related to high mortality and long ICU stay time (3). Therefore, more attention should be focused on the postoperative nosocomial pneumonia to improve clinical outcomes.
Surveillance for nosocomial pneumonia is a critical mission in mechanically ventilated patients. For the reason that VAP is morbid, common and preventable, quality of care has traditionally been measured by using VAP rates (4). Nevertheless, some significant shortcomings of VAP surveillance have hindered its application in quality improvement programs. Firstly, a gold standard for VAP diagnosis is absent. Clinical diagnosis for VAP has always been relied on the National Healthcare Safety Network pneumonia definition (5), which is proven to be neither sensitive nor specific. Tejerina and colleagues demonstrated that half of patients who fulfilled this diagnostic criterion were actually not infected with pneumonia according to the histopathological evidence at autopsy (6). Some key items such as “new or progressive infiltrates” or “worsening gas exchange” are rather subjective for clinicians, and some clinical information such as “new onset or worsening cough, dyspnea and tachypnea” could be impossibly observed in sedated patients, thus leading to an increased number of false positive or false negative diagnoses. Furthermore, some other conditions during mechanical ventilation (MV) that also reflect the deterioration in gas exchange cannot be included by using VAP surveillance, such as pulmonary edema, atelectasis, bronchitis, acute respiratory distress syndrome (ARDS) and so on. All these factors bring urgent demands for new comprehensive surveillance.
Recently, a working group was convened by the Centers for Disease Control and Prevention (CDC) to propose a solution for all above mentioned limitations. An innovative definition, ventilator-associated event (VAE), was then proposed to replace VAP surveillance in ventilated patients. Three tiers are included in VAE surveillance, which are ventilator-associated condition (VAC), infection-related ventilator-associated complication (IVAC), probable or possible VAP. The new definition is thought to reorient the focus from pneumonia alone to all infectious or non-infectious conditions caused by MV, defined as respiratory deterioration after a period of stable or improved gas exchange. Compared with previous VAP definition, this program eliminates the difficulty in diagnosis and broadens the surveillance spectrum to multiple complications encompassing pneumonia (7).
Although there is a rich literature on clinical characters of VAP, few information about VAE can be acquired, especially in patients who have undergone heart operation. In this retrospective study, we collected 1,709 patients, and further analyzed the prevalence, risk factors, etiology and clinical outcomes of VAE after cardiac surgery for the first time, aiming at providing a comprehensive description of this new surveillance algorithm.
Methods
Definitions
According to the CDC’s criteria, the definition of VAE is three tiered. The first tier is VAC, which is defined as an increase of at least 3 cmH2O in daily minimum positive end expiratory pressure (PEEP) or an increase of at least 20 points in daily minimum fraction of inspired oxygen (FiO2) over baseline for at least 2 days, after at least 2 days of stable or decreasing PEEP or FiO2. The second surveillance tier, IVAC, attempts to identify the subset of VAC that may be infection-related, as evidenced by concurrent inflammatory signs and the initiation of new antimicrobial agents. The third tier is possible VAP or probable VAP. Possible VAP definition requires the presence of purulent secretions or positive pulmonary cultures. Probable VAP definition requires purulent secretions plus positive quantitative or semi-quantitative cultures for pathogenic organisms (8) (Figure 1).

Study design
This retrospective study was performed at the department of cardiovascular surgery in Chengdu Military General Hospital, a tertiary-level medical center in Chengdu, China. Consecutive patients who have undergone cardiac surgery and were greater than 18 years old were included in the present study from September 2012 to December 2015 (our research was approved by Human Ethical Committee of Chengdu Military General Hospital, and the ID of the Ethic Approval was “2017ky10”.). Based on the above definitions, all patients were classified in either VAE group or non-VAE group.
Data extraction
By reviewing electronic medical records and preserved files, the following clinical characteristics were extracted: age, gender, left ventricle ejection fraction (LVEF), New York Heart Association (NYHA) grading, pulmonary hypertension, chronic obstructive pulmonary disease (COPD), peptic ulcers, diabetes, CPB time, aortic clamping time, intra-aortic balloon counterpulsation (IABP), acute kidney injury (AKI), re-intubation, infectious endocarditis, blood products, MV time, ICU stay time, total hospitalization time. Pulmonary hypertension is defined as pulmonary artery systolic pressure over 30 mmHg based on tricuspid regurgitation measured by echocardiography. COPD is diagnosed according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) document (9). Acute Kidney Injury Network (AKIN) criteria is used to define AKI after cardiac surgery (10). Blood products refer to red blood cells, fresh-frozen plasma, platelets and others which were postoperatively administrated until VAE was established.
Microbiological samples
Samples from the respiratory tract were routinely obtained from patients receiving MV for more than 48 h. Firstly, we put a sterile catheter into the endotracheal tube. Once in position, suction was applied. Then the aspirates were collected immediately into the trap and sent to be inspected within 2 h. Samples were considered positive when the bacterial count was greater than 105 colony forming unit per milliliter (CFU/mL) for each specific pathogen. All microorganisms were identified using standard method. Polymicrobial infections were considered as episodes with more than one microorganism isolated in respiratory secretions.
Statistics
The results were analyzed by using software SPSS version 20.0. Descriptive data were expressed as means ± standard deviation (SD) or percentages. Student’s t-test was used for normally distributed continuous variables. Chi-squared test or Fisher’s exact test was performed to compare categorical variables. In addition, multiple logistic regression analysis was applied to assess independent contribution of predictor variables with odds ratios (OR) and 95% confidence intervals (CI). All statistical tests were two tailed, and P<0.05 was considered significant.
Results
Patient characteristics
We totally included 1,709 adult patients in the present research, with a median of 46.7 MV hours. Valve replacement or plasty operation is the most common surgical type, accounting for 68.3 percent of total, followed by coronary artery bypass graft (CABG) and great blood vessel operation. We also concluded the information about age, gender, pulmonary hypertension, COPD, peptic ulcers, LVEF <30%, NYHA grade IV, CPB time, aortic clamping time and mortality, which were listed in Table 1.
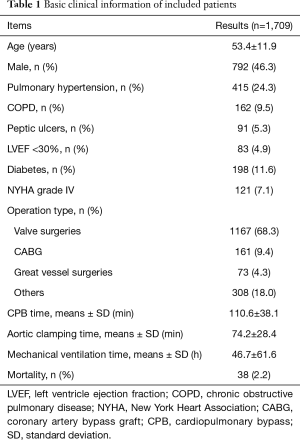
Full table
Prevalence
Among the 1,709 patients, a total of 166 episodes (9.7%) met the CDC’s criteria for VAE, of which VAC developed in 25 patients (15.1%), IVAC developed in 46 patients (27.7%), and possible or probable VAP developed in 95 patients (57.2%). The mean VAE incidence rate was 49.9 per 1,000 MV days. In 269 patients ventilated for at least 4 consecutive days, the prevalence of VAE was up to 61.7%. As shown in Table S1, we concluded that VAP was the leading cause, followed by bloodstream infection, tracheobronchitis and so on. Among the non-infectious events, cardiac arrest was most closely related to VAEs.
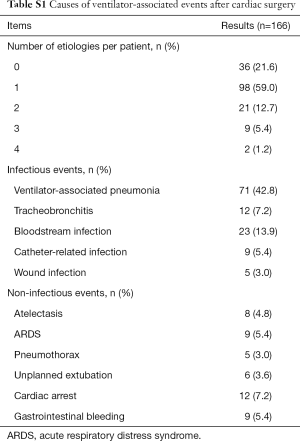
Full table
Risk factors
Table 2 showed that 8 of 17 variables were associated with an increased risk for VAE by using univariate analysis. Preoperative risk factors were verified to be COPD (23.5% vs. 8.0%, P<0.01), LVEF<30% (12.0% vs. 4.1%, P<0.01), NYHA grade IV (13.9% vs. 6.4%, P<0.01). Intraoperative CPB time (121.3±31.6 vs. 109.5±38.6 min, P<0.01) and aortic clamping time (79.3±25.4 vs. 72.6±28.7 min, P<0.05) could also significantly affect the occurrence of VAE. For postoperative risk factors, the following were associated with VAE: MV time (146±63 vs. 36±51 h, P<0.01), reintubation (13.9% vs. 5.3%, P<0.01) as well as dosage of blood products (6.7±3.1 vs. 5.5±3.6 units, P<0.01).
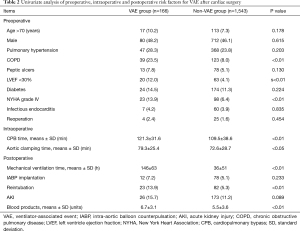
Full table
In addition, nine factors with P<0.1 in the univariate analysis were further tested by logistic regression analysis (Table 3). It was observed that only NYHA grade IV was excluded (OR: 1.863, 95% CI: 0.913–2.894, P=0.092), while the other eight factors were identified as the significant independent predictors for VAE: COPD (OR: 3.841, 95% CI: 1.031–76.828, P<0.05), LVEF<30% (OR: 1.811, 95% CI: 1.107–2.973, P<0.05), CPB time (OR: 4.849, 95% CI: 2.317–9.851, P<0.01), aortic clamping time (OR: 4.392, 95% CI: 1.078–18.132, P<0.05), MV time (OR: 25.144, 95% CI: 8.512–50.310, P<0.01), reintubation (OR: 3.667, 95% CI: 1.309–11.142, P<0.05), dosage of blood products (OR: 19.654, 95% CI: 7.338–41.368, P<0.01), AKI (OR: 4.014, 95% CI: 2.075–26.781, P<0.05).
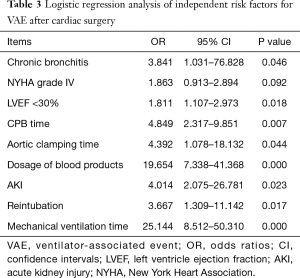
Full table
Clinical outcomes
The results about clinical outcomes were summarized in Table 4. VAE was proved to be associated with a longer length of stay in ICU. Mean ICU stay time was up to 13.3 days in VAE group, and 3.1 days in non-VAE group (P<0.01). Duration of hospitalization was also longer in patients with VAE than in patients without VAE (29.4±14.5 vs. 19.7±8.3 days, P<0.01). Once patients were monitored for VAEs, mortality was significantly increased (9.0% vs. 1.5%, P<0.01).

Full table
Etiology
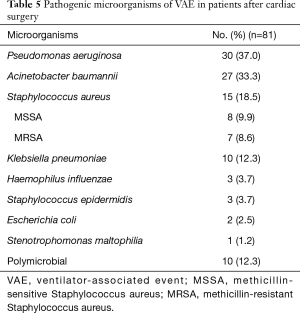
As Table 5 showed, 91 strains of pathogens were isolated from endotracheal aspirates of 81 patients with VAE. We found that VAE was polymicrobial in ten patients (12.3%). Pseudomonas aeruginosa was the most common pathogenic microorganism (30 isolates, 37.0%), followed by Acinetobacter baumannii (27 isolates, 33.3%). Among 15 Staphylococcus aureus isolates (18.5%), there were 8 methicillin-sensitive Staphylococcus aureus (MSSA) and 7 methicillin-resistant Staphylococcus aureus (MRSA). Some other bacteria were also examined: Klebsiella pneumonia (10 isolates, 12.3%), Haemophilus influenza (3 isolates, 3.7%), Staphylococcus epidermidis (3 isolates, 3.7%), Escherichia coli (2 isolate, 2.5%).
Full table
Discussion
On account of the dependence on subjective clinical signs and radiographic evidence, VAP surveillance is always criticized for the lack of reliability and validity. Recently, CDC has put forth a new surveillance definition for VAE to replace VAP in adults (8). VAE framework contains a hierarchy of definitions: VAC, IVAC, probable or possible VAP. This new term covers all the conditions that result in deterioration in oxygenation, which is supposed to broaden the scope of surveillance and to make surveillance more objective. Instead of giving the diagnostic information to inform immediate clinical management, VAE surveillance is just intended to provide an overall estimate of complication rates (11).
The incidence rates of VAE reported in previous literature has great differences. By using the same VAE definition and adjusting the denominator to all MV patients, the prevalence is considered to range from 4.0% to 5.8% and 11.1 to 13.8 episodes per 1,000 MV days (12). In the present study, the rate of VAE in postoperative patients is up to 9.7% and 49.9 episodes per 1,000 MV days, which is much higher than previous results. It is probably for the reason of surgical strike, especially CPB, that has impaired lung function. For patients undergoing cardiac surgery, CPB could easily trigger inflammatory responses and ischemia reperfusion injuries (13), leading to the release of inflammatory cytokines and oxygen-free radicals, tissue infiltration by neutrophils, activation of reactive oxygen species, and so on (14). All these adverse factors contribute to postoperative pulmonary dysfunction, thus increasing the risks of infection. In addition, the VAE rate reported in this research is almost the twice of VAP rate (6.37% and 21.27 cases per 1,000 MV days) that was concluded in our previously published meta-analysis (3), demonstrating the wider surveillance scope of the former.
As with VAP, VAE is also consistent with poor prognosis, including longer ICU stay time and hospitalization time as well as higher mortality, which was verified in the present study. For the relative frequency of occurrence and attributable adverse impacts, effective strategies are eagerly demanded for clinicians to prevent VAEs. However, there is still scant evidence for prevention. Boyer and his colleagues gave an estimate that only 37.3% of VAEs could be preventable (15). So, a number of clinicians have focused their attention on risk factors in order to explore potential targets for intervention. By conducting a retrospective case control study, Lewis et al. found that mandatory modes of ventilation and fluid status are risks for VAC while initiating benzodiazepines prior to intubation, opioid exposures and paralytic medications are risks for IVAC (16). Another retrospective cohort study demonstrated immunocompromised status, tracheostomy dependence and chronic respiratory disease are risk factors for VAE but this research was completed in a pediatric ICU (17). According to the results of our study, eight factors were screened from 17 candidates and were verified as the risks for VAE. There was a discrepancy about the result of NYHA grade IV between univariate analysis and multiple logistic regression analysis, which we suppose is probably caused by the relative subjectivity of NYHA grading. Clearly, these results provide important information to us about the targeted prevention. For example, we could endeavour accelerating the operation progress and shortening the time of CPB and aortic clamping, minimize duration of MV, avoid unplanned weaning and reintubation, and reduce the transfusion of blood products. Furthermore, patients in conditions that cannot be interfered, such as preoperative COPD and LVEF <30%, postoperative AKI, and so on, should be treated with special care. In addition to our reports, many experts have also obtained achievements to some degree concerning the intervention measures. Klompas has put forward a perspective that VAEs are generally caused by 1 of 4 conditions: pneumonia, fluid overload, atelectasis, and ARDS (18). So, any interventions targeting these specific conditions should be the potential approaches for prevention, including enhancing performance of coordinated spontaneous awakening trials (SATs) and spontaneous breathing trials (SBTs), avoiding intubation, minimizing sedation, early exercise and mobility, low tidal volume ventilation, conservative fluid management, and so on (18,19). Some of these potential strategies have been gradually verified in prospective interventional analyses. A recent clinical trial suggested that paired daily SATs and SBTs could significantly lower VAE rates, and was closely associated with shorter ventilation, ICU stay and hospitalization time (20). By increasing concordance with multiple VAP prevention recommendations, VAP and VAC rates were found to be significantly reduced in spite of no changes in IVAC rate (21). During weaning process, a depletive fluid management strategy was also proven to decrease the occurrence of VAC and VAP (22). Nevertheless, another issue we should interpret with caution is that not all VAP prevention bundles can be used to prevent VAE. One randomized controlled trial revealed that subglottic secretion suctioning resulted in a significant reduction of VAP prevalence, but had no influence on VAE rates (23).
Given that VAE is a surveillance for nosocomial infection, choosing an appropriate antimicrobial regimen seems rather critical. In the present study, we firstly focused on the microbiological pathogens of VAEs, and demonstrated that Pseudomonas aeruginosa and Acinetobacter baumannii were the two major causes of VAEs, together accounting for over half of total. When worsening oxygenation begins after at least 2 days of stable ventilation, VAE could easily occur. At this moment, we recommend using antibiotics against these two types of pathogens as empirical therapy, such as third or fourth generation cephalosporins, until drug sensitivity results come out. Initial treatment of proper antimicrobial program is probably beneficial to prevention or associated with well prognosis, but this speculation needs to be further validated in future studies.
There still exist some limitations in our study. First, this is a retrospective research performed in a single medical unit, which could not represent the general information in other cardiac centers. Second, different human reviewers were assigned to collect data from previous electronic medical records and preserved files instead of by using computed database. Manual surveillance may increase the unreliability a lot compared with an automated surveillance. Third, as the substitution of VAP, VAE surveillance is considered to be more valid, but the relationship between these two algorithms is not explored in the present research. At last, although we analyzed the risk factors for VAEs and gave several potential strategies for prevention, effective intervention measures were still not yet determined in the patients undergoing heart operations. It is difficult to estimate preventability retrospectively. So more prospective studies on interventional approaches are encouraging in the future.
Conclusions
It is the first study that provides a detailed description of VAE in patients who have undergone cardiac surgeries. By analyzing clinical data retrospectively, all trials that met the criteria of three tiered VAE surveillance were screened. We observed that VAE had high incidence in this subpopulation, almost the twice of VAP rates. Once patients were enrolled in this VAE surveillance scope, poor prognosis would consistently occur, including high mortality, prolonged ICU stay time and hospitalization time. Pseudomonas aeruginosa was the most common pathogenic microorganism, followed by Acinetobacter baumannii and others. Subsequently, we further found out eight risk factors for VAE by using both univariate analysis and multiple logistic regression analysis, intending to give potential strategies for prevention.
Acknowledgements
Funding: This work was supported by the Project of Youth Scientific and Technological Innovation in Chengdu Military General Hospital (grant number: 41732C11K).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by Human Ethical Committee of Chengdu Military General Hospital (ID: 2017ky10).
References
- Allou N, Bronchard R, Guglielminotti J, et al. Risk factors for postoperative pneumonia after cardiac surgery and development of a preoperative risk score*. Crit Care Med 2014;42:1150-6. [Crossref] [PubMed]
- O'Grady NP, Murray PR, Ames N. Preventing ventilator-associated pneumonia: does the evidence support the practice? JAMA 2012;307:2534-9. [PubMed]
- He S, Chen B, Li W, et al. Ventilator-associated pneumonia after cardiac surgery: a meta-analysis and systematic review. J Thorac Cardiovasc Surg 2014;148:3148-55.e1-5.
- Klompas M. Ventilator-associated events surveillance: a patient safety opportunity. Curr Opin Crit Care 2013;19:424-31. [Crossref] [PubMed]
- Canadian Critical Care Trials G. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med 2006;355:2619-30. [Crossref] [PubMed]
- Tejerina E, Esteban A, Fernandez-Segoviano P, et al. Accuracy of clinical definitions of ventilator-associated pneumonia: comparison with autopsy findings. J Crit Care 2010;25:62-8. [Crossref] [PubMed]
- Raoof S, Baumann MH, Critical Care Societies Collaborative, et al. Critical Care Societies Collaborative, et al. Ventilator-associated events: the new definition. Am J Crit Care 2014;23:7-9. [Crossref] [PubMed]
- Magill SS, Klompas M, Balk R, et al. Developing a new, national approach to surveillance for ventilator-associated events*. Crit Care Med 2013;41:2467-75. [Crossref] [PubMed]
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [Crossref] [PubMed]
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [Crossref] [PubMed]
- Septimus E, Green L, Klompas M. Ventilator-associated events: a broader perspective. Crit Care Med 2015;43:e59-61. [Crossref] [PubMed]
- Zhu S, Cai L, Ma C, et al. The Clinical Impact of Ventilator-Associated Events: A Prospective Multi-Center Surveillance Study. Infect Control Hosp Epidemiol 2015;36:1388-95. [Crossref] [PubMed]
- Asimakopoulos G, Smith PL, Ratnatunga CP, et al. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg 1999;68:1107-15. [Crossref] [PubMed]
- Huffmyer JL, Groves DS. Pulmonary complications of cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol 2015;29:163-75. [Crossref] [PubMed]
- Boyer AF, Schoenberg N, Babcock H, et al. A prospective evaluation of ventilator-associated conditions and infection-related ventilator-associated conditions. Chest 2015;147:68-81. [Crossref] [PubMed]
- Lewis SC, Li L, Murphy MV, et al. Risk factors for ventilator-associated events: a case-control multivariable analysis. Crit Care Med 2014;42:1839-48. [Crossref] [PubMed]
- Phongjitsiri S, Coss-Bu J, Kennedy C, et al. The Centers for Disease Control and Prevention's New Definitions for Complications of Mechanical Ventilation Shift the Focus of Quality Surveillance and Predict Clinical Outcomes in a PICU. Crit Care Med 2015;43:2446-51. [Crossref] [PubMed]
- Klompas M. Potential Strategies to Prevent Ventilator-associated Events. Am J Respir Crit Care Med 2015;192:1420-30. [Crossref] [PubMed]
- Chahoud J, Semaan A, Almoosa KF. Ventilator-associated events prevention, learning lessons from the past: A systematic review. Heart Lung 2015;44:251-9. [Crossref] [PubMed]
- Klompas M, Anderson D, Trick W, et al. The preventability of ventilator-associated events. The CDC Prevention Epicenters Wake Up and Breathe Collaborative. Am J Respir Crit Care Med 2015;191:292-301. [Crossref] [PubMed]
- Muscedere J, Sinuff T, Heyland DK, et al. The clinical impact and preventability of ventilator-associated conditions in critically ill patients who are mechanically ventilated. Chest 2013;144:1453-60. [Crossref] [PubMed]
- Mekontso Dessap A, Katsahian S, Roche-Campo F, et al. Ventilator-associated pneumonia during weaning from mechanical ventilation: role of fluid management. Chest 2014;146:58-65. [Crossref] [PubMed]
- Damas P, Frippiat F, Ancion A, et al. Prevention of ventilator-associated pneumonia and ventilator-associated conditions: a randomized controlled trial with subglottic secretion suctioning. Crit Care Med 2015;43:22-30. [Crossref] [PubMed]


