Pleurectomy/decortication versus extrapleural pneumonectomy: a critical choice
Introduction
Malignant pleural mesothelioma (MPM) is a relatively rare and aggressive tumor which arises from the mesothelial pleural cells, and is commonly associated with asbestos exposure (1). Currently, there are approximately 3,000 cases per year in the United States, and in Europe its incidence is about 20 cases per million, with large variation between the different countries (2,3). MPM’s peak of incidence in most developed counties is expected between 2010 and 2020 (4,5).
MPM is associated with an extremely poor prognosis: a median survival of less than 1 year and a 5-year survival rate of less than 2% are commonly observed (6). The optimal management for MPM is still debated, but up to now, cisplatin and pemetrexed-based chemotherapy (CT) is considered the standard of care, since randomized controlled trial (RCT) have reported its superiority in terms of survival and quality of life, compared to cisplatin alone (7,8). Administration of induction/adjuvant systemic CT has become the standard of care in case of any surgical procedure with curative intent (9).
The role of surgery continues to be controversial. It is very difficult, in fact, to choose the subset of patients who may benefit from a surgery-based multimodal treatment, compared to those in which CT alone or CT plus radiotherapy (RT) is offered with a palliative intent.
Surgery for MPM usually may include both minor palliative procedures (VATS/small thoracotomy pleural biopsies with or without talc poudrage) and more aggressive ones [extrapleural pneumonectomy (EPP) and pleurectomy/decortication (P/D)].
The aim of this paper is to critically evaluate indications and results of both EPP and P/D, according to the MPM clinical presentation.
The goals of surgery
Surgery can help to achieve a MPM correct diagnosis (open or VATS pleural biopsies) or to palliate symptoms caused by malignant pleural effusions (VATS talc pleurodesis, VATS pleurectomy, indwelling pleural drainage placement). In very selected patients (good performance status and early-stage disease) “radical surgical procedures” have been offered in the past, with or without different induction/adjuvant treatments (10,11).
Whenever aggressive surgery is planned, its aim is to remove all gross disease, lengthening survival by reducing the intrathoracic tumor burden to microscopic levels. Due to MPM’s particular biological characteristics, the intra-thoracic anatomical structures may be rapidly invaded by the tumor, generally without development of distant metastases. This loco-regional tumor invasiveness should be taken into account when a surgical approach is planned. MPM surgery per se is a maximal cytoreductive procedure, and usually results in a R1 resection. A complete resection (R0) with surgery alone is theoretically unobtainable, regardless the type of aggressive procedure is chosen.
Therefore, the first goal of surgery is to achieve a macroscopic complete resection (MCR); other treatment modalities have been combined with surgery, with the aim to control the residual microscopic disease. Surgery alone has no role in MPM treatment; patients should undergo this treatment in the setting of a multidisciplinary tumor board, where the single clinical cases are presented and carefully discussed. Ideally, all candidates for surgery should be operated in tertiary hospitals, in Thoracic Surgery Units whit recognized great experience in MPM management. Those units are commonly connected with Medical and Radiation Oncology Divisions also involved in clinical trials for MPM.
Recently, Flores and colleagues (12) have reported that radical surgery is offered in the United States in approximately 22% of patients with MPM, while the figures may be lower in Europe and in developing countries, due to the lack of local expert and the limited trend to refer patients to tertiary hospitals.
The secondary goal for surgery is to achieve a tumor local control (evacuate pleural effusions, palliate chest pain caused by possible chest wall invasion, free an entrapped lung and ameliorate ventilation/perfusion mismatch).
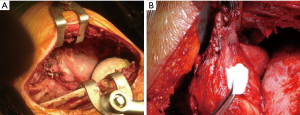
There are two surgical approaches to achieve MCR in MPM patients: (I) lung-sacrificing and (II) lung-sparing operations. The first is the “EPP”, the en bloc resection of the lung, parietal pleura, pericardium and diaphragm, with the subsequent diaphragmatic and pericardial prosthetic reconstruction (13) (Figure 1A). The P/D surgical technique was not initially standardized in all Centres, being able to vary from minimally-invasive (VATS) partial pleural resection, with a palliative intent, to a radical excision, involving also the pericardium and the hemidiaphragm. To carry out a proper uniformity of P/D definition, the International Association for the Study of Lung Cancer (IASLC) has recently published a Consensus Report, which definitively classifies P/D into three categories (14):
- Extended P/D: resection of the parietal and visceral pleurae, to remove all gross tumor, associated with resection of the hemidiaphragm and/or pericardium;
- P/D: parietal and visceral pleura resection to remove all gross tumor, without resection of pericardium and/or hemidiaphragm;
- Partial pleurectomy: partial resection or parietal and/or visceral pleurae for palliative intent, only, leaving gross tumor behind.
EPP
Historically, the use of EPP has been initially reported by Butchart and colleagues in 1976. The Authors described 29 consecutive patients who underwent pleuro-pneumonectomy for MPM: the in-hospital mortality was 31% and 3 patients, only, survived 2 years or longer (15). Taking into account this high mortality and the limited outcome, this treatment has been stopped for many years. Nevertheless, the development of modern surgical and anaesthetics techniques, along with the recent dramatic improvement in oncologic MPM management, have significantly reduced both postoperative complications and mortality, local and distant tumor relapses and improved long-term outcome. In 1999, Sugarbaker et al. have published a large series of MPM patients treated with EPP (11). The reported in-hospital mortality was lower and the 5-year survival rate was up to 46% in very selected patients (MPM epithelioid histological subtype, clear surgical margins and absence of lymph-nodal involvement). Therefore, several Authors started again to offer this operation to patients with epithelial MPM, very often in combination with induction CT and adjuvant RT. EPP, in fact, seemed to be the best operation to allow postoperative hemithoracic radiation to treat residual microscopic disease, without concerns for possible pulmonary toxicity.
Moreover, the recent development of intrapleural hyperthermic cisplatin-based CT, led selected specialist Centres to improve survival by reducing the risk of loco-regional tumor relapses (16,17).
The role of EPP in the treatment of MPM has been recently debated, after the publication of the Mesothelioma and Radical Surgery (MARS I) trial results (18). The trial provocative conclusions (“EPP within trimodality therapy offers no benefit and possibly harms patients”) caused a long series of debates into the scientific community, although 16 patients, only, were included in the EPP arm. The trial was, in fact, dedicated to patients whose MPM extent and general performance status allowed the option to receive induction CT and, if successfully completed, to be randomized between EPP versus no surgical intervention. To be realistic, the study was not designed to give an answer to the question if EPP could be detrimental or not, but to the feasibility of such a trial, only. Indeed, as Weder and colleagues reported (19), an accrual of 670 patients would be required to give a definitive answer to this question.
P/D
P/D has been described as treatment option for MPM since 1976, when Wanebo and colleagues reported a median survival of 21 months for patients treated with pleurectomy followed by RT and adjuvant CT (20). Pleurectomy extent may vary according to the tumor burden and surgeon’s type of surgical resection preference: therefore, a comparison between different types of pleurectomy becomes very difficult. This is the reason why a 3-level pleurectomy description has been recently proposed by the IASLC (14).
The results of recent studies report extended P/D less perioperative morbidity and mortality compared to EPP (21-24): therefore, some authors conclude that P/D is superior to EPP in term of overall outcome. However, as emphasized by Flores et al. (25), caution should be used in these results interpretations. The authors, in fact, observe that: (I) several surgeons do not consider EPP and extended P/D to be interchangeable; and (II) for selected patients (e.g., those with a bulky disease or those in which MPM also involve the fissures), EPP should be considered as the only surgical option which may offer a real MCR.
Administering adjuvant RT after P/D, with the lung still in situ, may cause severe toxicity and high risk of radiation pneumonitis (26). Grades 3–4 pneumonitis have been reported in up to 20% of patients (24). Nevertheless, extended P/D, especially when a trimodality scheme is scheduled, is associated with a better short-term outcome, compared to EPP (27,28). Intra-postoperative P/D most common complications are: bleeding (sometimes more profuse than in EPP), postoperative persistent air leaks [caused by the visceral pleura resection (Figure 1B)] and mucous plugging with possible atelectasis.
Perioperative mortality (2.9% after P/D vs. 6.8% after EPP; P=0.02) as well as perioperative morbidity (27.9% after P/D vs. 62.0% after EPP; P<0.0001) are found to be significantly lower after extended P/D compared to EPP (29). Also, QoL seems to be better after extended P/D: pulmonary function, for example, improves within few weeks after operation (30,31).
Finally, the presence of both the lungs allows the patients to complete adjuvant CT and better tolerate the proposed treatment (usually CT and/or RT) in case of tumor recurrence.
Conclusion: which operation is best?
MPM is considered an orphan disease and surgery is offered in less than 20% of patients: this fact makes the total denominator for patients receiving surgery-based treatment extremely small, and therefore, it is really difficult to design a RCT able to give a clear answer to this question.
However, it is recognized that acceptance and QoL are better after P/D than after EPP. The median survival is better in P/D group (16 months P/D vs. 12 months EPP), as reported by Flores and colleagues in a retrospective review of 633 patients from three Institutions (25). A recent meta-analysis of outcome after P/D and EPP has confirmed that EPP patients have a significantly higher proportion of short-term mortality (4.5% EPP vs. 1.7% P/D, P<0.005) and complications, compared to P/D, while there has no statistical difference in 2-year mortality between the two groups (23.8% EPP vs. 25% P/D, P=0.8) (32). Furthermore, patients receiving P/D are able to better tolerate adjuvant CT by the simple fact of having two lungs. Nevertheless, tumor recurrences in the ipsilateral chest are two times higher in the P/D group, compared to EPP.
Since thoracic CT scan and MRI are generally inaccurate in defining MPM local extent, surgeons are frequently unable to decide the most appropriate surgical operation until the time of thoracotomy. Therefore, they must be ready to perform both EPP or P/D; this choice should be done based on the tumor’s extent and the patient’s functional status.
The ideal scenario for P/D is a small amount of disease, limited to the parietal pleura with small foci of visceral pleura involvement. When visceral and parietal pleura surfaces are fused and the tumor invades the underlying lung, EPP is generally required to resect all gross disease. P/D may provide a R1 resection and it seems to be a suboptimal surgical procedure for the tumor at this stage. In fact, as Pass and colleagues have demonstrated, there is a significant relationship in term of overall survival, between the preoperative tumor extent and the residual gross disease after surgery (33): surgeons, therefore, should be prepared to perform either operations based on the intraoperative findings. EPP is required in case of advanced stage disease, when all pleural surfaces, as well as diaphragm and pericardium are involved, and there may be tumor extension into the endothoracic fascia or to the mediastinal tissue.
MPM patients should be clearly informed of the surgical options, in order to achieve an informed consent. In absence of a clear evidence showing an EPP survival benefit, some Authors assume that this operation should be offered only in selected high experienced Centres, ideally in ongoing prospective clinical trials, and the outcome carefully evaluated (23).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Park EK, Takahashi K, Hoshuyama T, et al. Global magnitude of reported and unreported mesothelioma. Environ Health Perspect 2011;119:514-8. [Crossref] [PubMed]
- Hodgson JT, McElvenny DM, Darnton AJ, et al. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer 2005;92:587-93. [Crossref] [PubMed]
- Lianes P, Remon J, Bover I, et al. SEOM guidelines for the treatment of malignant pleural mesothelioma. Clin Transl Oncol 2011;13:569-73. [Crossref] [PubMed]
- Robinson BM. Malignant pleural mesothelioma: an epidemiological perspective. Ann Cardiothorac Surg 2012;1:491-6. [PubMed]
- Frost G. The latency period of mesothelioma among a cohort of British asbestos workers (1978-2005). Br J Cancer 2013;109:1965-73. [Crossref] [PubMed]
- Ceresoli GL, Locati LD, Ferreri AJ, et al. Therapeutic outcome according to histologic subtype in 121 patients with malignant pleural mesothelioma. Lung Cancer 2001;34:279-87. [Crossref] [PubMed]
- Nowak AK. Chemotherapy for malignant pleural mesothelioma: a review of current management and a look to the future. Ann Cardiothorac Surg 2012;1:508-15. [PubMed]
- Kondola S, Manners D, Nowak AK. Malignant pleural mesothelioma: an update on diagnosis and treatment options. Ther Adv Respir Dis 2016;10:275-88. [Crossref] [PubMed]
- Cao C, Tian D, Manganas C, et al. Systematic review of trimodality therapy for patients with malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:428-37. [PubMed]
- Maziak DE, Gagliardi A, Haynes AE, et al. Surgical management of malignant pleural mesothelioma: a systematic review and evidence summary. Lung Cancer 2005;48:157-69. [Crossref] [PubMed]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63. [Crossref] [PubMed]
- Flores RM, Riedel E, Donington JS, et al. Frequency of use and predictors of cancer-directed surgery in the management of malignant pleural mesothelioma in a community-based (Surveillance, Epidemiology, and End Results [SEER]) population. J Thorac Oncol 2010;5:1649-54. [Crossref] [PubMed]
- Rusch VW. Extrapleural pneumonectomy and extended pleurectomy/decortication for malignant pleural mesothelioma: the Memorial Sloan-Kettering Cancer Center approach. Ann Cardiothorac Surg 2012;1:523-31. [PubMed]
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol 2011;6:1304-12. [Crossref] [PubMed]
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [Crossref] [PubMed]
- Tilleman TR, Richards WG, Zellos L, et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J Thorac Cardiovasc Surg 2009;138:405-11. [Crossref] [PubMed]
- Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955-63. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Weder W, Stahel RA, Baas P, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093-4. [Crossref] [PubMed]
- Wanebo HJ, Martini N, Melamed MR, et al. Pleural mesothelioma. Cancer 1976;38:2481-8. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Belcher E, et al. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine followed by adjuvant chemotherapy in patients with malignant pleural mesothelioma. J Thorac Oncol 2011;6:1746-52. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:737-43. [Crossref] [PubMed]
- Lang-Lazdunski L. Surgery for malignant pleural mesothelioma: why, when and what? Lung Cancer 2014;84:103-9. [Crossref] [PubMed]
- Wolf AS, Flores RM. Current Treatment of Mesothelioma: Extrapleural Pneumonectomy Versus Pleurectomy/Decortication. Thorac Surg Clin 2016;26:359-75. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6. [Crossref] [PubMed]
- Lee TT, Everett DL, Shu HK, et al. Radical pleurectomy/decortication and intraoperative radiotherapy followed by conformal radiation with or without chemotherapy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2002;124:1183-9. [Crossref] [PubMed]
- Burt BM, Cameron RB, Mollberg NM, et al. Malignant pleural mesothelioma and the Society of Thoracic Surgeons Database: an analysis of surgical morbidity and mortality. J Thorac Cardiovasc Surg 2014;148:30-5. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J Thorac Cardiovasc Surg 2016;151:478-84. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Bölükbas S, Eberlein M, Schirren J. Prospective study on functional results after lung-sparing radical pleurectomy in the management of malignant pleural mesothelioma. J Thorac Oncol 2012;7:900-5. [Crossref] [PubMed]
- Tanaka T, Morishita S, Hashimoto M, et al. Physical function and health-related quality of life in patients undergoing surgical treatment for malignant pleural mesothelioma. Support Care Cancer 2017;25:2569-75. [Crossref] [PubMed]
- Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472-80. [Crossref] [PubMed]
- Pass HI, Temeck BK, Kranda K, et al. Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1998;115:310-7. [Crossref] [PubMed]


