Red cell distribution width is associated with hospital mortality in unselected critically ill patients
Introduction
Red cell distribution width (RDW) is a measurement of the variability of red cell sizes and it increases in response to inflammatory stimulation or poor nutritional status (1,2). RDW has been linked to clinical outcomes in varieties of clinical settings. For instance, Nathan SD and coworkers (3) found that RDW is an independent predictor of outcome in patients with idiopathic pulmonary fibrosis; similar results have been replicated in many other diseases and settings including acute coronary syndrome, patients undergoing cardiopulmonary bypass surgery, community acquired pneumonia, acute cerebral infarction and bacteremia (4-7). These results render RDW a promising biomarker for risk stratification of patients.
In critical care setting, it is of vital importance to stratify patients on their entry to intensive care unit (ICU). This will help to make full use of the limited ICU resources, inform the anxious patients’ relatives and institute appropriate medical treatment. Thus, continuous efforts have been made to find models or biomarkers that can provide information on the prognostication of critically ill patients. There are generally two types of methods used for the prediction of patients’ mortality. One includes varieties of scores such as APACHE scores, SOFA and simplified acute physiology score (SAPS); the other includes biomarkers such as C-reactive protein (CRP) and soluble urokinase plasminogen activator receptor (8,9). Due to its promising results obtained in other clinical settings, RDW has also been investigated in ICU. However, these results are preliminary and further confirmations are needed. The present study aimed to investigate the prognostic value of RDW in critically ill patients. We hypothesized that (I) RDW measured on entry to ICU is predictive of the in-hospital mortality; (II) repeated measurements of RDW during ICU stay is of limited value due to the long lifespan of red blood cells.
Methods
The study was conducted in a 24-bed mixed ICU of a tertiary teaching hospital. This hospital had 1,980 open beds and provided medical care for over five million people in Jinhua region. Patients’ electronic information was recorded in Haitai e-chart (provided by Haitai Medical information systems CO., LTD, Nanjing, P.R. China). These information included data on demographic characteristics, all laboratory findings, imaging studies and medications. The study was approved by IRB of our hospital. The study was approved by ethics committee of Jinhua municipal central hospital has therefore been performed in accordance with the ethical standards laid down in the Declaration of Helsinki, and ethics committee of Jinhua municipal central hospital waived the need for written informed consent from the participants due to the retrospective nature of the study.
All patients that had been treated in ICU from October 2009 to December 2012 were considered to be eligible. Patients who had their medical records incomplete, transferred to other hospitals during the course of treatment, or signed do-not-resuscitation order were excluded. Such patients were labeled as “automatically discharged” in the Haitai medical system.
Demographic data abstracted from e-chart included age, sex, diagnosis, use of mechanical ventilation (MV) and the use of continuous renal replacement therapy (CRRT). Laboratory data included hemoglobin, CRP, mean corpuscular volume (MCV) and albumin. These variables were measured on entry to ICU. RDW was included in the complete blood cell count (CBC) and was routinely measured for each patient treated in ICU. It was measured in automatic hematology analyzer. The normal reference of RDW in our laboratory was 11.6-14.8%. All consecutive measurements of RDW were abstracted from the database. Because physiology-based scores for the measurement of illness severity were not routinely recorded in our e-chart, we used the well validated Charlson comorbidity index for risk adjustment (10,11). Patients were followed during their hospital stay. The primary endpoint was in-hospital mortality and the secondary study endpoint was ICU length of stay (LOS). For patients readmitted to ICU within seven days, the ICU LOS was the sum of the two ICU LOSs; for patients readmitted to ICU beyond seven days, only the first ICU LOS was used for analysis.
Statistical analysis
Continuous variables were tested for normality by using skewness and kurtosis test. Normally distributed data were expressed as mean ± SD (standard deviation) and compared between groups by using t-test; skewed data were expressed as median and interquartile range (IQR) and tested using Wilcoxon rank-sum test. Demographic data and baseline variables were compared between survivors and nonsurvivors. Multivariable logistic regression model was used to screen independent variables that were associated with in-hospital mortality. Variables with P<0.1 in the univariate analysis were entered into the multivariable model. Hosmer-Lemeshow method was used to test the goodness-of-fit of the regression model. Diagnostic performance of CRP, RDW, albumin, Charlson index and their combination in discriminating survivors and nonsurvivors were evaluated by using receiver operating characteristic (ROC) curve (12). Logistic regression model was used to obtain coefficients (β) for each variable. Then a new variable Y was calculated according to the equation: Y= exp (β0+β1X1+β2X2+β3X3+… βnXn)/1+ exp (β0+β1X1+β2X2+β3X3+… βnXn); where X denotes each included variables, and in the present situation it refers to CRP, RDW, albumin and Charlson index. ICU LOS was compared between patients with RDW >14.8% and ≤14.8% by using Log-rank test. To exclude potential confounding effect of death on the analysis of ICU LOS, patients died in ICU were excluded from analysis (e.g., patients who died shortly after ICU entry appears to have a very short LOS in ICU, which will erroneously render this group of patients to have a good clinical outcome). Changes in RDW (∆RDW) were calculated on 3, 6, 10, 13, 17 and 20 days during ICU stay. Patients were divided into two groups with ∆RDW >0 or ≤0 to examine whether the changes in RDW during ICU stay were associated with in-hospital mortality. All statistical analyses were performed using StataSE 11.2 (College Station, Texas 77845 USA). Conventional two-tailed P<0.05 was considered to be statistically significant.
Results
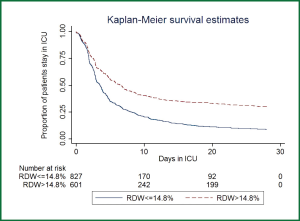
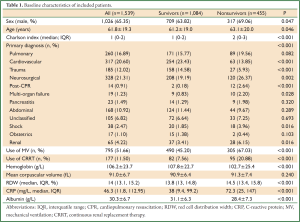
A total of 1,539 patients were eligible for the current analysis during study period. Demographic data and baseline characteristics were shown in Table 1. During their hospital stay, there were 1,084 survivors and 455 nonsurvivors. Male patients were more likely to die than female patients (69.06% vs. 63.82%, P=0.047). Nonsurvivors were significantly older than survivors (63.1 vs. 61.2 years, P=0.046). The Charlson indices were significantly higher in the nonsurvivors than survivors (median: 2 vs. 1, P<0.001). The primary diagnoses were not equally distributed between the two groups. There were more patients with primary diagnoses of cardiovascular diseases (23.43% vs. 13.85%, P<0.001), trauma (14.58% vs. 5.93%, P<0.001) in survivor group than in nonsurvivor group; conversely, there were more patients with the primary diagnoses of neurosurgical disorders (26.37% vs. 19.19%, P=0.002), post cardiac arrest (2.64% vs. 0.18%, P<0.001), multi-organ failure (2.2% vs. 0.83%, P=0.028), shock (3.96% vs. 1.85%, P=0.016) and renal failure (6.15% vs. 3.14%, P=0.016) in nonsurvivor group than survivor group. More patients in the nonsurvivor group used MV (67.03% vs. 45.20%, P<0.001) and CRRT (20.88% vs. 7.56%, P<0.001) than survivor group. Hemoglobin levels were significantly higher in the survivor group than that in the nonsurvivor group (107.8±22.7 vs. 102.7±25.4 g/L, P<0.001). MCVs were similar between survivors and nonsurvivors. RDWs were significantly higher in nonsurvivors (14.5% vs. 13.8%, P<0.001). Figure 1 displays the Kaplan-Meier survival estimates for the ICU LOS in patients with RDW >14.8% and ≤14.8%. The result showed that patients with RDW >14.8% had significantly longer LOS in ICU (Log-rank test, P<0.001).
Full table
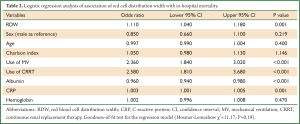
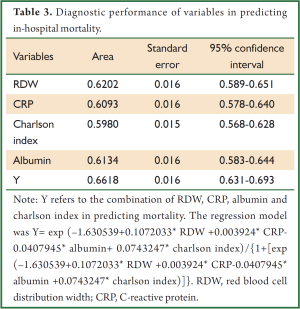
In multivariable model, RDW was independently associated with in-hospital mortality (OR: 1.1, P=0.002). Other variables including albumin, CRP, use of MV and CRRT were also independently associated with mortality (Table 2). The regression model was well fitted as demonstrated by Hosmer-Lemeshow χ2=11.17 (P=0.19). Variables independently associated with in-hospital mortality were assessed for their ability in discriminating survivors and nonsurvivors. However, there individual discriminating abilities were suboptimal and of limited clinical utility. The areas under ROC curve (AU-ROC) for RDW, CRP, Charlson index and albumin were 0.62, 0.61, 0.60 and 0.61, respectively (Table 3 and Figure 2). The combination of these variables significantly improved the discriminating power, but slightly in magnitude (AUC =0.66, 95% CI: 0.631-0.693).
Full table
Full table
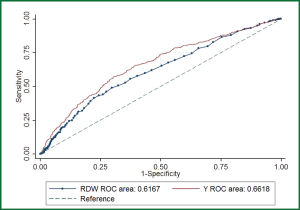
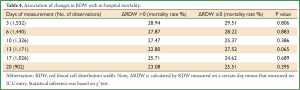
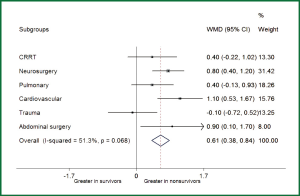
Table 4 shows the association of changes in RDW with in-hospital mortality. Repeated measurements of RDW were arbitrarily selected on day 3, 6, 10, 13, 17 and 20 during ICU stay. The results showed that none of these ∆RDWs was associated with in-hospital mortality, indicating that repeated measurements of RDW are of limited value in critical care setting. Post hoc subgroup analysis was performed to investigate whether the association of RDW with mortality differed across different ICU populations (Figure 3). The results showed that RDW was significantly higher in nonsurvivors than that in survivors in subgroups of cardiovascular disease (WMD: 1.1%; 95% CI: 0.53-1.67%), abdominal surgery (WMD: 0.9%; 95% CI: 0.1-1.7%) and neurosurgery (WMD: 0.8%; 95% CI: 0.4-1.2%). There was a trend towards higher RDW in nonsurvivors in subgroups of CRRT (WMD: 0.4%; 95% CI: –0.22-1.02%) and pulmonary diseases (WMD: 0.4%; 95% CI: –0.13-0.93%). However, in trauma patients, there was a trend towards higher RDW in survivors than nonsurvivors (WMD: –0.1%; 95% CI: –0.72-0.52%).
Full table
Discussion
Our study confirmed previous finding that RDW was associated with clinical outcomes in critically ill patients. Specifically, our study showed that higher RDW was associated with increased in-hospital mortality and prolonged ICU LOS. However, the ability of RDW in distinguishing survivors from nonsurvivors was suboptimal, and the repeated measurements of RDW offered no additional clinical value in predicting outcomes.
The first report on the association of RDW with clinical outcome in critically ill patients used ICU mortality as the primary study end point; as a result, patients were regarded to have a favorable outcome if they were successfully discharged from ICU (13). However, many patients die in floor ward after they are successfully discharged from ICU. Even when their illness deteriorates, these patients and their next-of-kin refuse to enter ICU again. Furthermore, the authors did not explicitly clarify the definition of ICU death in the situation of ICU readmission. In a recent study, Badawi O and coworkers found that ICU readmission was experienced by 3% of ICU patients (14). Thus, we feel that use of in-hospital mortality as the primary end point is more reasonable in the situation that patients are discharged prematurely due to limited ICU resources. However, the choice of study endpoint seems to have no impact on the result and our finding is consistent with that reported by Wang F and colleagues (13). In a large multicenter cohort study, Bazick HS and colleagues (15) also showed a strong association of RDW with all cause mortality at different follow-up time points. However, diagnostic statistics such as AUC, sensitivity and specificity were not reported in that study, which limited the clinical application of their results. Nonetheless, our study shows that the discriminating power of RDW is of limited clinical utility due to a very low AUC. RDW only adds slightly to the discriminating power of conventional variables such as Charlson index, CRP and albumin. Such low predictive value may be explained by the heterogeneity of the study population. In a study including only patients with septic shock, Sadaka F and colleagues (16) showed a much better discrimination of RDW for mortality. Further studies are needed to investigate whether analysis restricting to certain subgroup can improve the diagnostic performance of RDW. Furthermore, the discriminating power of RDW is confounded by other measured and unmeasured risk factors for mortality, including physiological disease severity scores, comorbidities and inflammatory biomarkers.
RDW is a quantitative measurement of anisocytosis and is calculated by dividing standard deviation of red blood cell volume with MCV. It increases in varieties of pathological conditions. The underlying mechanism of the association of RDW with mortality is largely unknown. However, several plausible hypotheses have been proposed, and inflammatory response and oxidative stress are among the most popular ones (17). Semba RD and colleagues (18) demonstrated that serum selenium level was an independent predictor of RDW, and this was mediated via interleukin-6 mediated inflammatory pathway. In our study, we found that the well known inflammatory biomarker CRP was also significantly increased in non-survivors. This is in support of the notion that increased inflammatory response is a harbinger of adverse clinical outcome (19,20). ICU patients are exposed to increased oxidative stress and inflammation response, both of which are responsible for the observed increase in RDW (21).
Another finding in the present study is that the measurement of RDW in a short interval offers no additional clinical value. This is reasonable since the lifespan of red blood cell is 120 days in normal population and even in pathological conditions the lifespan can only drop to 30 days (22). Thus, its change within 28 days (a study period that is commonly used in critical care setting) is not clinically meaningful. Repeated measurements may be more meaningful in chronic health conditions in which patients are followed for longer period of time. In a community-based study, Semba RD and colleagues (18) made a series of measurements of RDW over a period of 24 months. They found that serum selenium level was in linear correlation with RDW over time. In another study involving cardiac failure patients, Oh J and colleagues found that it was the change in RDW between hospital admission and one month after discharge that could predict cardiovascular events, while the change of RDW between admission and discharge had no significant association with cardiovascular events (23). Although the authors did not mention the erythrocyte lifespan as a potential explanation for this observation, we proposed that an interval of 30 days between two measurements of RDW may be necessary to make this clinically meaningful.
The results of subgroup analysis are generally in line with that reported in literature. For instance, Oh HJ and colleagues (24) reported that RDW was an independent predictor of 28-day mortality in patients with CRRT. In patients with cardiovascular diseases, there are numerous studies being published all consistently showing a significant link between RDW and mortality (25-27). The same result is replicated in our investigation. An interesting finding in the study is that RDW tends to be higher in survivors than non-survivors in trauma patients; although the low statistical power due to limited sample size may explain such conflicting finding; we proposed other possible explanations. First, trauma patients usually require large volumes of blood transfusion that will introduce large number of deformed erythrocytes (28,29). As a result, the first measurement of RDW on ICU entry appears to be increased because the patient receives liberal blood transfusion in emergency department. Probably, the higher RDWs can reflect adequate resuscitation with packed RBCs, which results in higher survival rate. Secondly, since RDW reflects the host inflammatory response (30,31), more intensive response may help to control or prevent subsequent infection during ICU stay. However, the result of this post hoc subgroup analysis is hypothesis-generating and further well designed investigations are needed to validate the result.
Our study contains several limitations. First, the proportion of patients with sepsis was unknown. This was due to the fact that the diagnosis of sepsis was not routinely recorded in our administrative database. Abstraction of data on sepsis from medical chart would distort the result. Second, the study is retrospective in nature, and bears the risk of potential bias. However, we tried to attenuate the risk by using multivariate analysis. Third, study population in the study is heterogeneous, which is a potential explanation for the low discriminating power of RDW as discussed previously.
Conclusively, in accordance with previous findings, the present study confirms that increased RDW measured on ICU entry is associated with higher hospital mortality. However, its diagnostic performance in predicting mortality is suboptimal. Repeated measurements of RDW in a short follow up period provide no additional value.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Förhécz Z, Gombos T, Borgulya G, et al. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J 2009;158:659-66. [PubMed]
- Lippi G, Targher G, Montagnana M, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med 2009;133:628-32. [PubMed]
- Nathan SD, Reffett T, Brown AW, et al. The red cell distribution width as a prognostic indicator in idiopathic pulmonary fibrosis. Chest 2013;143:1692-8. [PubMed]
- Massad MG, Abdelhady K. Red blood cell distribution width as a biomarker for need for coronary artery bypass graft surgery and its clinical outcome. Cardiology 2012;123:133-4. [PubMed]
- Lee JH, Chung HJ, Kim K, et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med 2013;31:72-9. [PubMed]
- Kim J, Kim YD, Song TJ, et al. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. Thromb Haemost 2012;108:349-56. [PubMed]
- Ku NS, Kim HW, Oh HJ, et al. Red blood cell distribution width is an independent predictor of mortality in patients with gram-negative bacteremia. Shock 2012;38:123-7. [PubMed]
- Giamarellos-Bourboulis EJ, Norrby-Teglund A, Mylona V, et al. Risk assessment in sepsis: a new prognostication rule by APACHE II score and serum soluble urokinase plasminogen activator receptor. Crit Care 2012;16:R149. [PubMed]
- Zhang Z, Ni H. C-reactive protein as a predictor of mortality in critically ill patients: a meta-analysis and systematic review. Anaesth Intensive Care 2011;39:854-61. [PubMed]
- Christensen S, Johansen MB, Christiansen CF, et al. Comparison of Charlson comorbidity index with SAPS and APACHE scores for prediction of mortality following intensive care. Clin Epidemiol 2011;3:203-11. [PubMed]
- Quach S, Hennessy DA, Faris P, et al. A comparison between the APACHE II and Charlson Index Score for predicting hospital mortality in critically ill patients. BMC Health Serv Res 2009;9:129. [PubMed]
- Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157-72; discussion 207-12. [PubMed]
- Wang F, Pan W, Pan S, et al. Red cell distribution width as a novel predictor of mortality in ICU patients. Ann Med 2011;43:40-6. [PubMed]
- Badawi O, Breslow MJ. Readmissions and death after ICU discharge: development and validation of two predictive models. PLoS One 2012;7:e48758. [PubMed]
- Bazick HS, Chang D, Mahadevappa K, et al. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med 2011;39:1913-21. [PubMed]
- Sadaka F, O’Brien J, Prakash S. Red cell distribution width and outcome in patients with septic shock. J Intensive Care Med 2013;28:307-13. [PubMed]
- Montagnana M, Cervellin G, Meschi T, et al. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med 2011;50:635-41. [PubMed]
- Semba RD, Patel KV, Ferrucci L, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women’s Health and Aging Study I. Clin Nutr 2010;29:600-4. [PubMed]
- Mygind ND, Iversen K, Køber L, et al. The inflammatory biomarker YKL-40 at admission is a strong predictor of overall mortality. J Intern Med 2013;273:205-16. [PubMed]
- Chua TC, Chong CH, Liauw W, et al. Inflammatory markers in blood and serum tumor markers predict survival in patients with epithelial appendiceal neoplasms undergoing surgical cytoreduction and intraperitoneal chemotherapy. Ann Surg 2012;256:342-9. [PubMed]
- Tozzi-Ciancarelli MG, Di Giulio A, Troiani-Sevi E, et al. Human erythrocyte damage at the initial stages of oxidative stress. Cell Biophys 1989;15:225-34. [PubMed]
- Krishnan SM, Dixit NM. Estimation of red blood cell lifespan from alveolar carbon monoxide measurements. Transl Res 2009;154:15-7. [PubMed]
- Oh J, Kang SM, Won H, et al. Prognostic value of change in red cell distribution width 1 month after discharge in acute decompensated heart failure patients. Circ J 2012;76:109-16. [PubMed]
- Oh HJ, Park JT, Kim JK, et al. Red blood cell distribution width is an independent predictor of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Nephrol Dial Transplant 2012;27:589-94. [PubMed]
- Anderson JL, Ronnow BS, Horne BD, et al. Usefulness of a complete blood count-derived risk score to predict incident mortality in patients with suspected cardiovascular disease. Am J Cardiol 2007;99:169-74. [PubMed]
- Tonelli M, Sacks F, Arnold M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008;117:163-8. [PubMed]
- Uyarel H, Ergelen M, Cicek G, et al. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis 2011;22:138-44. [PubMed]
- Sollberger T, Walter R, Brand B, et al. Influence of prestorage leucocyte depletion and storage time on rheologic properties of erythrocyte concentrates. Vox Sang 2002;82:191-7. [PubMed]
- Knight JA, Searles DA, Clayton FC. The effect of desferrioxamine on stored erythrocytes: lipid peroxidation, deformability, and morphology. Ann Clin Lab Sci 1996;26:283-90. [PubMed]
- Öztürk ZA, Ünal A, Yiğiter R, et al. Is increased red cell distribution width (RDW) indicating the inflammation in Alzheimer’s disease (AD)? Arch Gerontol Geriatr 2013;56:50-4. [PubMed]
- Ozcan F, Turak O, Durak A, et al. Red cell distribution width and inflammation in patients with non-dipper hypertension. Blood Press 2013;22:80-5. [PubMed]


