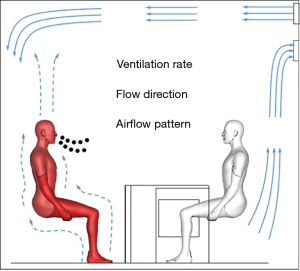
Ventilation control for airborne transmission of human exhaled bio-aerosols in buildings
Introduction
The possible serious threat of airborne infection in buildings to human health has been reiterated by the 2003 worldwide severe acute respiratory syndrome (SARS) epidemic (1), the growing potential threat of bio-terror attack through deliberately releasing agents such as anthrax or smallpox (2), H1N1 influenza epidemic in 2011 (3) and MERS in 2013 (4). The growing urbanization, the worsening overcrowded conditions in modern large cities and the rapidly growing worldwide transport networks are possibly making the transmission of airborne spread infectious diseases faster (5). Airborne transmission mostly occurred in indoor environments (5,6), where most people spend over 90% of their time.
Ventilation is recognized as an important factor influencing the transmission of airborne diseases. The significance of ventilation was also reemphasized by the 2003 worldwide SARS outbreak in 2003 worldwide and in particular a super spreading event in a hospital in Hong Kong. The investigation of the largest nosocomial SARS outbreak in Hong Kong in the Prince of Wales Hospital by Li et al. (2005) (7) and Tomlinson and Cockram (2003) (8) suggested that an inefficient ventilation system very probably caused the spread of the virus in the Ward 8A. Gao concluded that increasing ventilation rates in classrooms, offices, and homes is a relatively effective strategy for controlling airborne diseases in a large city through using the large urban contact networks calculation (6,9).
Ventilation is one of the most important means to control the cross infection by removing or diluting virus-laden aerosols exhaled by infected patients. Ventilation is defined as the supply/distribution or removal of air from space by mechanical or natural means (10). The purpose of ventilation is to supply outdoor air and remove extra heat, humidity and contaminants from occupied spaces to meet health and comfort requirements. Ventilation in hospitals is also expected to remove the droplets nuclei efficiently, which potentially contain pathogens, so as to minimize the cross infection risk and to supply pathogen-free fresh air for breathing (5). Different ventilation strategies may be required for patients with different diseases in a hospital. It is generally believed that for a general ward and a negative pressure isolation ward, the ideal ventilation system is to exhaust or dilute the contaminants timely and to supply pathogen-free fresh air to healthcare workers (HCWs) and inpatients efficiently. The airflow direction should be controlled properly from clean zones to dirty zones, preventing the virus-laden aerosols transmission between rooms.
The role of ventilation in preventing airborne infections has drawn extensive attention since the SARS outbreak. The mechanism of dispersion of airborne droplets/droplet nuclei in space, the risk estimation of airborne infection, the role of airflow rate, the impact of airflow pattern, and etc. have been comprehensively explored in previous studies. Herein, this paper is aimed to provide a basic insight into the transmission mechanism of airborne infection, the risk estimation of airborne infection and introduce three key elements of ventilation (i.e., ventilation rate, flow direction and airflow pattern, as shown in Figure 1) influencing the airborne infection.
The mechanism of airborne infection
The discovery of airborne diseases in humans has a long history. Up to 10,000 B.C., smallpox epidemics were reported in northeastern Africa (11). In the ancient time, it was believed that black magic or malevolence of witches caused the infection. The oldest “scientific” theories of spread of epidemic diseases considered that certain diseases were transmitted via air in the sixteenth century (12). It was suggested that diseases were caused by miasma and malaria in air, which referred to noxious vapors and bad air respectively (13,14). However, with the establishment of germ theory, the concepts of contact and droplet-borne infection became prevailing (14). Wells explained that the path of airborne transmission of diseases was via droplet nuclei and the hypothesis was subsequently verified by experiments (15-17). This concept of vehicles of pathogen airborne transmission has been widely accepted (14,18-20).
Pathogen-laden droplets, which are expelled into air while a patient sneezes, coughs, speaks, sings or simply breathes (21,22), subsequently dry out in the air and produce droplet nuclei, the fine particles that can suspend in air (23). There can be 40k to more than two million droplets released from a sneeze, compared to fewer than 100,000 from a cough, and 3,000 from loudly speaking (21,23). The mechanisms of respiratory droplets generation and releasing from mucus to mouth were reviewed by Wei (24).
Droplets of less than 100 m in diameter were found to evaporate very rapidly (17,25-27), leaving behind residue particles consisting of the dried solute (such as sodium chloride) and any other solid matter contained in the original droplet. Droplet nuclei may also contain organisms presented in the original droplets which may not be damaged by the drying and dehydration process and can potentially infect a susceptible host. The sizes of those droplet nuclei are generally less than 5 m and may be readily inhaled into the lungs. Fennelly et al. (28) first cultured the M. tuberculosis (TB) from the droplet nuclei exhaled from patients with active TB. They also found that for a patient coughing naturally (not induced), the infectious particular size was 2.1–3.3 m.
These droplet nuclei can possibly contain organisms originally present after the evaporation, which are protected by a coat of dry secretions and suspend indefinitely in air and transport over a long distance (29). Once particles are inhaled, larger particles will be taken out from the upper respiratory tract. They will impinge on hairs, ciliated surfaces or mucosae, and finally be captured since they have more inertia and are less able to change direction quickly. On the contrary, smaller particles with little inertia can be carried wherever the air goes. Droplet nuclei can deeply penetrate into bronchi and deposit in lungs, where the air motion is minimal (20).
Wells carried out an experiment to study the retention of noninfectious particles of different sizes by using infectious particles (16). He exposed rabbits to the air with a known concentration of droplet nuclei containing single bovine tubercle bacilli (settling velocity approximately 0.03 ft/min and aerodynamic size is about 2 to 3 microns). Similarly, he also exposed rabbits to contaminated coarser particles (settling velocity approximately 1 ft/min and aerodynamic diameter about 12 microns) produced by the atomized concentrated fluid. Through calculation, he found that the number of tubercles developing in the rabbits' lungs approximately equaled that of inhaled live tubercle bacilli when rabbits were exposed to the fine particles, while only about 6% of these potentially infectious particles produced tubercles when rabbits were exposed to the coarse particles. Results indicated that most of the infectious droplet nuclei were trapped in lungs while only a small percentage of coarser particles reached the deep lungs. It demonstrated the significance of fine droplet nuclei in the airborne transmission of TB.
Transmission of infectious diseases occurs when the pathogen or agent leaves the source and spreads by one or more routes of transmission to the susceptible. Droplet spread and airborne transmission are two main routes to transmit respiratory diseases. Droplet spread refers to the passage of pathogens from a source to a susceptible through large droplets. It was calculated that droplets of greater than 100 m in diameter released from a height of 2 m deposited on the floor within 3–6 s with less than 1.5 m in horizontal distance at room air temperature and relative humidity of less than 60%, while droplets of less than 100 m evaporated within 3–6 s (17,25,26,30). So droplet-borne transmission is a short-range process, with distance less than 2 m due to the evaporation and high settling velocity of large droplets. While airborne transmission refers to the passage of pathogens from a source to a susceptible through airborne aerosols, resulting in infections (5). The vehicle of airborne transmission is droplet nuclei, the residues of dried-out droplets, which can suspend in air for a long time and transmit over a long distance. Liu et al. (31) investigated the interpersonal exposure of exhaled droplets and droplet nuclei between two standing thermal manikins affected by different factors, i.e., distance, temperature and humidity. He found that the risk of short-range airborne infection was much higher than that of long-range airborne infection when only the concentration of droplet nuclei was considered. Results also showed that the mechanisms of droplet-borne and short-range airborne infections are totally different, in spite that they are all short-range modes. Furthermore, the death rate of airborne microorganisms was found low after the first rapid death phase from atomizer when there was no disinfection method involved (32,33). Those characters indicated that the short-range transmission had a much higher risk than the long-range transmission does. The determination of droplet-borne or airborne infection should not be according to the transmitted range, i.e., 2 m. The mechanisms of ventilation for controlling human exhaled airborne aerosols are different for droplet-borne, short-range airborne and long-range airborne transmissions.
The impact of ventilation rate
Increasing ventilation rate is believed to reduce the cross infection of airborne transmitted diseases by removing or diluting pathogen-laden airborne droplet nuclei. A higher ventilation rate can dilute the contaminated air inside the space more rapidly and decrease the risk of cross infection. Menzies et al. studied the association between tuberculin conversion among HCWs and the ventilation rate in patient care areas (34). They found that the tuberculin conversion among HCWs was strongly associated with inadequate ventilation in general patient rooms and duration of work. Jiang et al. investigated the infection risk of HCWs in different wards with different window sizes in two hospitals during the 2003 SARS outbreak in Guangdong and found that larger ventilation windows showed a lower infection risk (35). In spite that a higher ventilation rate is able to provide a higher dilution capability to reduce the cross infection, the use of higher ventilation rates also means a higher energy cost for mechanical ventilation. However, there was a lack of strong scientific evidence in recommending a minimum ventilation flow rate for infection control according to Li et al.’s systemic review (5). The balance between decreasing the cross infection risk and reducing the energy consumption is needed. The recommended minimum ventilation rate for airborne infection isolation rooms is 12 air change per hour (ACH) in most guidelines (29,36,37), which is originated from the 6 ACH in Center for Disease Control and Prevention (CDC) guideline (38) and then doubled after the 2003 SARS, while it is only 1 ACH for commercial buildings.
The impact of ventilation rate on the cross infection of airborne transmitted diseases can be described by Wells-Riley Equation. Wells introduced an idea of quantal infection to describe the necessary dose of pathogens to cause infection to a new susceptible (15). Based on this assumption and Poisson distribution, the infection possibility was derived by Riley (39), which is called Wells-Riley equation, to predict the risk of airborne infection:
where P is the risk of cross infection, C is the number of case to develop infection, S is the number of the susceptible, I is the number of infectors, p is the pulmonary ventilation rate of each susceptible (m3/h), Q is the room airflow rate (m3/h), q is the quanta produced by one infector (quanta/h), and t is the duration of exposure (h).
The Wells-Riley equation successfully predicted a measles outbreak in a suburban school in USA (39). The equation and its improvements have been widely used to predict outbreaks of airborne infections and even to study the association between sick leave and ventilation system (40-43). The equation indicates that ventilation rate can reduce the infection risk significantly.
Natural ventilation is able to deliver large ventilation rates with a low energy consumption. Compared with mechanical ventilation, natural ventilation can provide much higher ventilation rates. Escombe et al. released the earliest publication of suggesting the use of natural ventilation for infection control (44). They studied different wards in eight hospitals ventilated in Lima and Peru, and found that natural ventilation could provide much larger ventilation rates than mechanical ventilation system did, which helped reduce the airborne contagion, especially with the high ceiling and large windows according to the calculation results of Wells-Riley equation. Qian et al. measured ventilation rates in two hospitals, whose wards were naturally ventilated in Hong Kong (45). Two hospitals are located in downtown and green areas respectively. Results showed that natural ventilation delivered a ventilation rate as high as 69 ACH for cross ventilation and 18 ACH for single-side ventilation when windows were fully open. The hospital located in green had a much higher potential of natural ventilation. Maximizing the natural ventilation has already been suggested for infection control in resource-limited regions (46). WHO published a guideline and introduced the usage of natural ventilation for infection control in healthcare settings (47). Six typical naturally-ventilated hospital wards were discussed in the guideline for reference, i.e., single-side corridor, central corridor, courtyard, wind tower, atrium and chimney, and hybrid (mixed-mode) ventilation. Zhou et al. evaluated the performance of different natural ventilation types in hospital wards by numerical simulation, finding that the central corridor ventilation, which has been broadly used in China, has the potential risk to cause cross infections between wards, indicating that it might not be recommended (48).
Based on previous studies, it is obvious that increasing the ventilation rate can reduce the risk of cross infection for long-range airborne infections. The impact of ventilation rate on the long-range airborne infection risk has been deeply explored. However, for droplet-borne transmitted diseases, the effect of ventilation does not seem so obvious. The fate of droplets is more governed by gravity and exhalation velocity. Ventilation can influence the evaporate rate by air flow and relative humidity. The impact of ventilation rate on the risk of short-range airborne diseases still needs further investigation.
The impact of flow direction
Flow direction can control contaminants transport among wards with different functions. Different ventilation strategies may be required for patients with different diseases in hospital. The ideal ventilation system for a general ward or a negative pressure isolation ward is expected to exhaust or dilute contaminants timely and to supply pathogen-free fresh air to HCWs and inpatients efficiently, Properly controlling the airflow direction from clean zones to dirty zones is of great importance to prevent the virus-laden aerosols transmitting between rooms.
The establishment of directional flow between zones is achieved through the pressure difference. The protective environment (PE) isolation room utilizes a positive pressure difference to resist the entry of surrounding contaminated air and thus avoid the infection of immune-compromised patients inside. On the other hand, the AII isolation rooms utilize a negative pressure difference to prevent the droplet nuclei generated by infected patients spreading to other zones. However, the negative pressure is only maintained when doors and windows are fully closed. When an isolation cubicle door is open, the negative pressure difference between cubicle and corridor will disappear (49-51). An anteroom was suggested or required by some guidelines to separate the ward and corridor (36,37).
The proper airflow direction is first from corridor to anteroom then to ward then to toilet. The pressure difference can be established through the imbalance of airflow rates. Different requirements of pressure difference or imbalance of airflow rate by different guidelines or design books range from 2.5 to 15 Pa (29,36,37,52,53). The required pressure difference of those guidelines seems to be empirical. Li (Personal Communication) considered that the pressure difference should be used to avoid the bi-directional flow which occurs due to temperature difference and wind force. He also calculated the minimum pressure difference to achieve the uni-direction flow and found that the required pressure difference was 0.36 and 9.12 Pa for temperature difference only and for combination of wind and temperature respectively, when temperature was 10 °C and the height of the door was 2.2 m. Hang employed computational fluid dynamics (CFD) simulations and full-scale experiments to evaluate potential inter-cubicle airborne transmissions through a shared anteroom due to the hinged door opening (49). Decreasing the duration of door opening, raising the air change rate or using a curtain at the doorway were recommended to reduce the inter-cubicle exposure risk.
It should be noted that real engineering construction quality may not realize the design goal. Hong Kong government promptly constructed 558 new negative pressure SARS isolation rooms with more than 1,300 beds in 14 hospitals in 2003. Those isolation rooms were designed according to international guidelines (29,36,37) and represented state-of-art technologies in 2003. The principle of designing the new SARS wards was also supported by CFD simulations and a full-scale test room study which were completed by the most experienced heating, ventilation and air conditioning (HVAC) experts of Hong Kong Institute. However, Li et al. field measured the ventilation performance of 38 SARS wards in 9 hospitals after 18 months of operation. And they found that 60% of toilets/bathrooms were operated under wrong airflow directions in spite that most wards met the recommended negative pressure difference of 2.5 Pa between corridor and anteroom (97%), and between anteroom and cubicle (89%) (54). Similar results were also reported by other field studies that up to 50% of the tested isolation rooms failed to provide a negative pressure (55-60). The main factors that broke up the negative pressure included inadequate reliability of pressure monitoring and controlling devices, strong diffuser flow directed at the door, interaction with other exhaust ventilation systems and poor airtightness of the suspended false ceiling (57).
The controlled flow direction is aimed at preventing cross infections between different wards or cubicles, indicating that it is an effective method to avoid the long-range airborne transmission among rooms. The methods are commonly used in hospital for controlling the cross infection. In normal commercial offices, the controlled flow direction is generally designed to create a positive pressure in rooms and a negative pressure in toilets, whose goal is to control the indoor temperature and pollutants. The pressure differences among rooms are not controlled, which may be the reason for the SARS outbreak in a Hong Kong Metropole Hotel, where the index patient infected patients in 13 neighbor guest rooms (61). The index patient vomited in the corridor, which was at positive pressure compared with guest rooms (62).
The impact of airflow pattern
Li et al. organized a panel comprised of experts from different majors to review the impact of ventilation on airborne infections (5). Strong and sufficient evidences of association between airflow and infection spread were found. Smoking test, tracer gas technique, direct aerosol dispersion measurement or CFD simulation were used to test airflow patterns and they agreed well with the spatial distribution of secondary infection (7,63-68). Qian et al. integrated the Wells-Riley equation into CFD models (69). The predicted spatial risk distribution in 8A wards in Prince of Wales Hospital agreed well with the spatial infection distribution pattern of SARS cases, indicating that the role of airflow pattern was important for airborne transmission diseases (69).
Three kinds of ventilation systems were commonly used, i.e., mixing ventilation, downward ventilation and displacement ventilation, as shown in Figure 2. Mixing ventilation supplies air with high momentum to make temperature, pollutants distribute uniformly, which is most widely used. For example, the ventilation system in Ward 8A of Prince of Wales Hospital in Hong Kong where the largest nosocomial SARS outbreak occurred in 2003 was of the mixing type (7). Downward ventilation was recommended by several guidelines for isolation wards (29,36,37). The basic idea of a downward ventilation design is to supply cooler and heavier clean air from a ceiling diffuser with a low velocity. The heavier cold supply air is accelerated by negative thermal buoyancy to push airborne particles down and be removed at the floor level. The “laminar” streams of air are expected to minimize the cross infection risk in isolation wards with a downward ventilation system. This concept was developed from industrial clean rooms and applied in surgical operating rooms in hospitals (70). The laminar airflow was considered to be promising in isolation wards for its care of hospital patients with low resistance in a positive pressure isolation room (71). This might be the original idea for extending the downward ventilation to be recommended in isolation wards including infection wards. The engineering control for surgical operating rooms or for immune-compromised hospital patients is focused on supplying fresh air directly to the patient’s body (wound) or breathing zone, and prevent surrounding contaminated air infecting the wound. However, the control for airborne infection isolation rooms is to prevent exhaled pathogen-laden droplet nuclei dispersing in the room.

Qian et al. carried experiments and CFD simulations to study the interaction of the breathing flows between two individuals in different ventilation systems and evaluated the performance of ventilation systems in removing exhaled pollutants from infected patients (72,73). Results showed that the exhaled jet penetrated a short distance and exhaled droplet nuclei were diluted quickly and well mixed in the ward. Bed distance did not affect the personal exposure of the receiving patient. The results indicated that the performance of downward ventilation to remove exhaled pollutants was close to that of mixing ventilation. For displacement ventilation, when the infector faced up, the ventilation system showed a very high efficiency to remove exhaled pollutants. However, when the infector faced horizontally, the exhaled jet could penetrate for a long distance and a high concentration layer of exhaled pollutants was found due to the thermal stratification lock up phenomena, which certainly added the risk of short-range airborne infection transmission. And if the height of lock up layer was located in the breathing zone, the risk of long-range airborne transmission would also be high. Zhou et al. theoretically derived the governing equations of jet in uniform and thermally-stratified environment and verified the model by experiment data in literature (74). The length of exhaled jet and height of lock up layer can be predicted, which is associated with temperature gradient, exhaled momentum, and exhaled temperature difference with ambient air. Large temperature gradient (usually in displacement ventilation) and great momentum of exhaled jet enlarge the spreading distance of short-range airborne transmitted diseases, which brings a higher risk in short-range airborne transmission than in long-range airborne transmission. Results indicated that displacement ventilation might not be used in hospital wards for preventing airborne risk.
Qian and Li developed an improved downward ventilation system to show a better performance to remove fine droplet nuclei (75). They compared the ventilation performances when exhausts were at different levels using full-scale experiments and CFD simulations. Results suggested that upper-level exhausts were more efficient than floor-level and near-head exhausts in removing gaseous contaminants due to upward body plumes. The low temperature air was supplied vertically from top and accelerated by gravity to deliver fresh air to HCWs directly, while the exhaust grill was also arranged at the top of ward to remove the up-flowing exhaled fine droplet nuclei. The mechanism of removing large particles is due to deposition instead of ventilation. The significance of surface cleaning is then approved.
Personalized ventilation (PV) may be another choice (24). PV with different terminal devices has been developed for delivering personal fresh air to improve local air quality and increase the occupant's satisfaction. However, it cannot remove exhaled droplet nuclei from the infector efficiently. Based on PV system, a novel system, PV-personalized exhaust (PV-PE), was developed for airborne infection control. Yang investigated three PV-PE systems, a top-personalized exhaust which is just above the human head, a shoulder-personalized exhaust which consists of two local exhausts installed at the chair just above the shoulder level, and a chair-personalized exhaust which acts as the upper part of chair (76). He found that all the three personalized exhaust devices could reduce the cross infection between occupants. The best one is the combination of a vertical desk grill and a top-personalized exhaust. Similar results were further approved and interaction with different ventilation systems were studied (77,78). Zheng et al. also developed a PV-PE system for inpatients (79). They set the personal air supply at the breathing level and exhaust at the top. Results showed that it had a much higher efficiency than traditional central air conditioning systems did. All studies revealed that the top exhaust may be more efficient in removing exhaled droplet nuclei due to the plume generated by human.
Conclusions
The impact of ventilation on the airflow pattern has been extensively studied. Ventilation is a useful engineering means to control airborne infection but it may not be an efficient way to control droplet-borne transmission. Higher ventilation rate is proved to reduce the risk of airborne infection, however, there is still a lack of scientific evidence of minimum ventilation rate. Short-range airborne transmission has a much higher risk, compared with long-range airborne transmission. Thermal stratification may extend the range of short-range airborne infection. The displacement ventilation is not suggested for isolation rooms. The performance of downward ventilation is very close to that of mixing ventilation due to interaction between upward plume and exhaled jet. An improved downward ventilation system was proposed. Properly using PV-PE systems can reduce the risk of airborne infection significantly.
Acknowledgements
Funding: The work described in this paper was funded by the Natural Science Foundation of China under the Project no. 51378103.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhong NS, Zheng BJ, Li YM, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet 2003;362:1353-8. [Crossref] [PubMed]
- Balali-Mood M, Moshiri M, Etemad L. Medical aspects of bio-terrorism. Toxicon 2013;69:131-42. [Crossref] [PubMed]
- Van Kerkhove MD, Vandemaele KA, Shinde V, et al. Risk factors for severe outcomes following 2009 influenza a (h1n1) infection: a global pooled analysis. PLoS Med 2011;8. [Crossref] [PubMed]
- Kim CJ, Choi WS, Jung Y, et al. Surveillance of the Middle East respiratory syndrome (MERS) coronavirus (CoV) infection in healthcare workers after contact with confirmed MERS patients: incidence and risk factors of MERS-CoV seropositivity. Clin Microbiol Infect 2016;22:880-6. [Crossref] [PubMed]
- Li Y, Leung GM, Tang JW, et al. Role of ventilation in airborne transmission of infectious agents in the built environment - a multidisciplinary systematic review. Indoor Air 2007;17:2-18. [Crossref] [PubMed]
- Gao X, Wei J, Lei H, et al. Building ventilation as an effective disease intervention strategy in a dense indoor contact Network in an ideal city. PLoS One 2016;11. [Crossref] [PubMed]
- Li Y, Huang X, Yu IT, et al. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air 2005;15:83-95. [Crossref] [PubMed]
- Tomlinson B, Cockram C. SARS: experience at Prince of Wales Hospital, Hong Kong. Lancet 2003;361:1486-7. [Crossref] [PubMed]
- Gao X, Wei J, Cowling BJ, et al. Potential impact of a ventilation intervention for influenza in the context of a dense indoor contact network in Hong Kong. Sci Total Environ 2016;569-70:373-81. [Crossref] [PubMed]
- Kreider JF, Rabl A, Curtiss P. Heating and cooling of buildings: design for efficiency. New York: McGraw-Hill, 1994.
- Snodgrass ME. World epidemics: a cultural chronology of disease from prehistory to the era of SARS. London,: McFarland & Company, 2003.
- Ayliffe GAJ, English MP. Hospital infection: from Miasmas to MRSA. Cambridge: Cambridge University Press, 2003.
- Langmuir AD. Epidemiology of airborne infection. Bacteriol Rev 1961;25:173-81. [PubMed]
- Langmuir AD. Airborne infection: how important for public health? I. A historical review. Am J Public Health Nations Health 1964;54:1666-8. [Crossref] [PubMed]
- Wells WF. On the mechanics of droplet nuclei infection .1. apparatus for the quantitative study of droplet nuclei infection of animals. Am J Hyg 1948;47:1-10. [PubMed]
- Wells WF, Ratcliffe HL, Crumb C. On the mechanics of droplet nuclei infection .2. quantitative experimental airborne tuberculosis in rabbits. Am J Hyg 1948;47:11-28. [PubMed]
- Wells WF. On air-borne infection study II. droplets and droplet nuclei. Am J Hyg 1934;20:611-18.
- Shaffer JG. 3. Airborne Infection in Hospitals. Am J Public Health Nations Health 1964;54:1674-82. [Crossref] [PubMed]
- Wedum AG. Ii. Airborne Infection in the Laboratory. Am J Public Health Nations Health 1964;54:1669-73. [Crossref] [PubMed]
- Riley RL, O'Grady F. Airborne infection: transmission and control. New York: The Macmillan Company, 1961.
- Duguid JP. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. J Hyg (Lond) 1946;44:471-9. [Crossref] [PubMed]
- Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med 1997;10:105-16. [Crossref] [PubMed]
- Cole EC, Cook CE. Characterization of infectious aerosols in health care facilities: An aid to effective engineering controls and preventive strategies. Am J Infect Control 1998;26:453-64. [Crossref] [PubMed]
- Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am J Infect Control 2016;44:S102-8. [Crossref] [PubMed]
- Wei J, Li Y. Enhanced spread of expiratory droplets by turbulence in a cough jet. Build Environ 2015;93:86-96. [Crossref]
- Xie X, Li Y, Chwang AT, et al. How far droplets can move in indoor environments - revisiting the Wells evaporation-falling curve. Indoor Air 2007;17:211-25. [Crossref] [PubMed]
- Xie X, Li Y, Sun H, et al. Exhaled droplets due to talking and coughing. J R Soc Interface 2009;6:S703-14. [Crossref] [PubMed]
- Fennelly KP, Martyny JW, Fulton KE, et al. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am J Respir Crit Care Med 2004;169:604-9. [Crossref] [PubMed]
- CDC. Guidelines for environmental infection control in health-care facilities. Available online: https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines.pdf
- Liu L, Wei J, Li Y, et al. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air 2017;27:179-90. [Crossref] [PubMed]
- Liu L, Li Y, Nielsen PV, et al. Short-range airborne transmission of expiratory droplets between two people. Indoor Air 2017;27:452-62. [Crossref] [PubMed]
- Ferry RM, Maple TG. Studies of the loss of viability of stored bacterial aerosols. I. Micrococcus candidus. J Infect Dis 1954;95:142-59. [Crossref] [PubMed]
- Harper GJ. Airborne micro-organisms: survival tests with four viruses. J Hyg (Lond) 1961;59:479-86. [Crossref] [PubMed]
- Menzies D, Fanning A, Yuan L, et al. Hospital ventilation and risk for tuberculous infection in canadian health care workers. Canadian Collaborative Group in Nosocomial Transmission of TB. Ann Intern Med 2000;133:779-89. [Crossref] [PubMed]
- Jiang S, Huang L, Chen X, et al. Ventilation of wards and nosocomial outbreak of severe acute respiratory syndrome among healthcare workers. Chin Med J (Engl) 2003;116:1293-7. [PubMed]
- AIA. Guidelines for design and construction of hospital and health care facilities. Washington: American Institute of Architects, Academy of Architecture for Health., Facilities Guidelines Institute, 2006.
- ASHRAE. HVAC design manual for hospitals and clinics. 2nd Edition. Atlanta: American Society of Heating Refrigerating and Air-Conditioning Engineers Inc., 2013.
- Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities, 1994. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/mmwr/pdf/rr/rr5417.pdf
- Riley EC, Murphy G, Riley RL. Airborne spread of measles in a suburban elementary-school. Am J Epidemiol 1978;107:421-32. [Crossref] [PubMed]
- Beggs CB, Noakes CJ, Sleigh PA, et al. The transmission of tuberculosis in confined spaces: an analytical review of alternative epidemiological models. Int J Tuberc Lung Dis 2003;7:1015-26. [PubMed]
- Rudnick SN, Milton DK. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air 2003;13:237-45. [Crossref] [PubMed]
- Fennelly KP, Davidow AL, Miller SL, et al. Airborne infection with Bacillus anthracis - from mills to mail. Emerg Infect Dis 2004;10:996-1002. [Crossref] [PubMed]
- Seppänen OA, Fisk WJ. Summary of human responses to ventilation. Indoor Air 2004;14:102-18. [Crossref] [PubMed]
- Escombe AR, Oeser CC, Gilman RH, et al. Natural ventilation for the prevention of airborne contagion. Plos Med 2007;4. [Crossref] [PubMed]
- Qian H, Li YG, Seto WH, et al. Natural ventilation for reducing airborne infection in hospitals. Build Environ 2010;45:559-65. [Crossref]
- WHO. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. 20 Avenue Appia, 1211 Geneva 27, Switzerland: World Health Organization, 2003.
- WHO. Natural ventilation for infection control in health-care settings. 20 Avenue Appia, 1211 Geneva 27, Switzerland: World Health Organization, 2009.
- Zhou Q, Qian H, Ren H. Numerical study of potential of natural ventilation for reducing airborne infections. 12th REHVA World Congress CLIMA; Aalborg, Denmark 2016:S634.
- Hang J, Li YG, Ching WH, et al. Potential airborne transmission between two isolation cubicles through a shared anteroom. Build Environ 2015;89:264-78. [Crossref]
- Adams NJ, Johnson DL, Lynch RA. The effect of pressure differential and care provider movement on airborne infectious isolation room containment effectiveness. Am J Infect Control 2011;39:91-7. [Crossref] [PubMed]
- Tang JW, Eames I, Li Y, et al. Door-opening motion can potentially lead to a transient breakdown in negative-pressure isolation conditions: the importance of vorticity and buoyancy airflows. J Hosp Infect 2005;61:283-6. [Crossref] [PubMed]
- SCICDHS. Guidelines for the classification and design of isolation rooms in health care facilities. Victoria, Australia: Standing Committee on Infection Control Department of Human Services, 1999.
- TSHRAE. Guidelines for design of air conditioning of respiratory infectious diseases isolation ward. Taiwan Society of Heating Refrigerating and Air-Conditioning Engineers 2003.
- Li Y, Ching WH, Qian H, et al. An evaluation of the ventilation performance of new SARS isolation wards in nine hospitals in Hong Kong. Indoor Built Environ 2007;16:400-10. [Crossref]
- Fraser VJ, Johnson K, Primack J, et al. Evaluation of rooms with negative pressure ventilation used for respiratory isolation in seven midwestern hospitals. Infect Control Hosp Epidemiol 1993;14:623-8. [Crossref] [PubMed]
- Dahl KM, L'Ecuyer PB, Jones M, et al. Follow-up evaluation of respiratory isolation rooms in 10 midwestern hospitals. Infect Control Hosp Epidemiol 1996;17:816-8. [Crossref] [PubMed]
- Pavelchak N, DePersis RP, London M, et al. Identification of factors that disrupt negative air pressurization of respiratory isolation rooms. Infect Control Hosp Epidemiol 2000;21:191-5. [Crossref] [PubMed]
- Pavelchak N, Cummings K, Stricof R, et al. Negative-Pressure monitoring of tuberculosis isolation rooms within New York State hospitals. Infect Control Hosp Epidemiol 2001;22:518-9. [Crossref] [PubMed]
- Sutton PM, Nicas M, Reinisch F, et al. Evaluating the control of tuberculosis among healthcare workers: adherence to CDC guidelines of three urban hospitals in California. Infect Control Hosp Epidemiol 1998;19:487-93. [Crossref] [PubMed]
- Rice N, Streifel A, Vesley D. An evaluation of hospital special-ventilation-room pressures. Infect Control Hosp Epidemiol 2001;22:19-23. [Crossref] [PubMed]
- Braden CR, Dowell SF, Jernigan DB, et al. Progress in global surveillance and response capacity 10 years after severe acute respiratory syndrome. Emerg Infect Dis 2013;19:864-9. [Crossref] [PubMed]
- Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air 2006;16:335-47. [Crossref] [PubMed]
- Bloch AB, Orenstein WA, Ewing WM, et al. Measles outbreak in a pediatric practice - airborne transmission in an office setting. Pediatrics 1985;75:676-83. [PubMed]
- Gustafson TL, Lavely GB, Brawner ER, et al. An outbreak of airborne nosocomial varicella. Pediatrics 1982;70:550-6. [PubMed]
- Hutton MD, Stead WW, Cauthen GM, et al. Nosocomial transmission of tuberculosis associated with a draining abscess. J Infect Dis 1990;161:286-95. [Crossref] [PubMed]
- Wehrle PF, Posch J, Richter KH, et al. An airborne outbreak of smallpox in a German hospital and its significance with respect to other recent outbreaks in Europe. Bull World Health Organ 1970;43:669-79. [PubMed]
- Yu ITS, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med 2004;350:1731-9. [Crossref] [PubMed]
- Wong TW, Lee CK, Tam W, et al. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis 2004;10:269-76. [Crossref] [PubMed]
- Qian H, Li YG, Nielsen PV, et al. Spatial distribution of infection risk of SARS transmission in a hospital ward. Build Environ 2009;44:1651-8. [Crossref]
- ASHRAE. 1999 HVAC applications handbook. Chapter 7: Health care facilities. .Atlanta, Ga.: American Society of Heating Refrigerating and Air-Conditioning Engineers Inc., 1999.
- Pfost JF. A re-evaluation of laminar air flow in hospital operating rooms. ASHRAE Trans 1981;87:729-39.
- Qian H, Li Y, Nielsen PV, et al. Dispersion of exhaled droplet nuclei in a two-bed hospital ward with three different ventilation systems. Indoor Air 2006;16:111-28. [Crossref] [PubMed]
- Qian H, Li Y, Nielsen PV, et al. Dispersion of exhalation pollutants in a two-bed hospital ward with a downward ventilation system. Build Environ 2008;43:344-54. [Crossref]
- Zhou Q, Qian H, Ren HG, et al. The lock-up phenomenon of exhaled flow in a stable thermally-stratified indoor environment. Build Environ 2017;116:246-56. [Crossref]
- Qian H, Li Y. Removal of exhaled particles by ventilation and deposition in a multibed airborne infection isolation room. Indoor Air 2010;20:284-97. [Crossref] [PubMed]
- Yang J, Sekhar C, Cheong KW, et al. CFD study and evaluation of different personalized exhaust devices. HVAC&R Res 2013;19:934-46. [Crossref]
- Yang J, Sekhar SC, Cheong KW, et al. Performance evaluation of a novel personalized ventilation-personalized exhaust system for airborne infection control. Indoor Air 2015;25:176-87. [Crossref] [PubMed]
- Yang JJ, Sekhar C, Cheong D, et al. Performance evaluation of an integrated Personalized Ventilation-Personalized Exhaust system in conjunction with two background ventilation systems. Build Environ 2014;78:103-10. [Crossref]
- Zheng XH, Qian H, Liu L. Numerical study on a new personalized ventilation system application in cross infection prevention. Journal of Central South University 2011;42:3905-11. (Science and Technology).