Sublobar resections for small-sized stage Ia lung adenocarcinoma: a Sino-Japanese multicenter study
Introduction
Lung cancer is the leading cause of cancer death worldwide for both men and women. Lobectomy with lymph node dissection remains the standard surgical procedure for early-stage non-small cell lung cancer (NSCLC). According to the only completed randomized study comparing lobectomy and sublobar resection for stage Ia NSCLC conducted in 1995, sublobar resection was associated with inferior overall survival, and three times higher local recurrence rate as compared to lobectomy (1). However, the conclusion of the LCSG study has recently been challenged for some issues, such as tumors of diameter >2 cm were involved in the study which might have caused the higher local recurrence rate in the sublobar arm (2).
In recent years, several retrospective reports have demonstrated that segmentectomy for small-sized (diameter ≤2.0 cm) stage Ia NSCLC may be comparable to lobectomy regarding prognosis and local recurrence (3-5). However, the clinical results of wedge resection for small-sized (diameter ≤2.0 cm) stage Ia NSCLC is still under debate (6,7). Therefore, it remains questionable whether wedge resection is oncologically inferior to segmentectomy for such patients. In this international multicenter study, we retrospectively studied the surgical outcomes of patients with small-sized (diameter ≤2.0 cm) stage Ia adenocarcinomas having sublobar resection to elucidate the role of sublobar resection and whether wedge resection is inferior to segmentectomy for such patients.
Methods
Patients
This retrospective study involved 354 consecutive patients of small-sized (diameter ≤2.0 cm) stage Ia adenocarcinomas receiving sublobar resection in Japan(National Cancer Center Hospital), and China (Shanghai Chest Hospital, and First Hospital of Tsinghua University) between January 2000 and August 2011. The study was approved by the Ethics Committee of the hospitals. Informed consent from patients was waived because of the retrospective nature of the study (Number/ID of the Ethic Approval is ks14025).
All patients were radiographically diagnosed as T1aN0M0 disease, and were operated on with a curative intent. High-resolution computed tomography (HRCT) scan was employed in all patients. Ground glass opacity (GGO) was defined as hazy increased lung attenuation without obscuring the underlying vascular marking and bronchial wall. All lesions were divided into two types, GGO type when the proportion of GGO was equal or more than 50% in HRCT, or solid type when the proportion of GGO was less than 50%.
Standard preoperative evaluation included pulmonary function testing with blood gas analysis, standard posterolateral chest films of chest, computed tomography of chest and brain, abdominal ultrasound, bone scintigraphy and positron emission tomography (PET) scan when necessary. The absence of nodal (mediastinal or hilar) disease and distant metastases was confirmed radiographically in all patients. Mediastinoscopy was not used in preoperative evaluation of stage Ia patients.
Of all patients, 173 (139 in Japan and 34 in China) underwent segmentectomy, and 181 (155 in Japan and 26 in China) underwent wedge resection. Sublobar resection was employed in 89 patients because of severe comorbidity or limited pulmonary function not adequate for lobectomy. Intentional sublobar resections were carried out in the rest of the patients. During sublobar resection, conversion to lobectomy was indicated while frozen-section of sampled lobar, hilar, and mediastinal lymph nodes was proved to be positive.
Statistical analysis
Statistical analysis was performed using SPSS 17.0. All continuous data was expressed as mean value ± standard deviation. Differences between groups with continuous variables were assessed by the χ2 test or Fisher’s exact test. Overall survivals and disease-free survivals were analyzed using Kaplan-Meier method and were statistically evaluated by log-rank test. Multivariate Cox regression analyses were performed to identify predictors of disease-free survival of all patients. Statistical significance was accepted as P<0.05 throughout the study.
Results
Between January 2000 and December 2011, 173 patients underwent segmentectomy (group I), and 181 patients underwent wedge resection (group II). Systemic lymphadenectomy or sampling was performed in segmentectomy, while sampling alone was carried out during wedge resection. Patients in Group I were younger than those in Group II, while the two groups were similar in sex and comorbidity rate. Percentage of Noguchi type A, B was higher in group II, while percentage of Noguchi type C, D, E was higher in group I. But the composition of Noguchi types was not of significant difference between the two groups (P=0.11).
The localization of the tumors is shown in Table 1. The superior segments of the lower lobes, and the upper division of the left upper lobe were the most common sites of segmentectomy. The average size of tumors was 1.3 cm in group I and 1.2 cm in group II respectively, without significant difference.
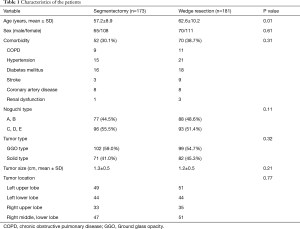
Full table
There was no in-hospital death in either group. Postoperative morbidity rate in group I was significantly higher than in group II (11.0% vs. 2.2%, P=0.016). Major complications of the two groups are listed in Table 2.
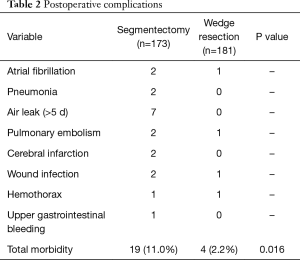
Full table
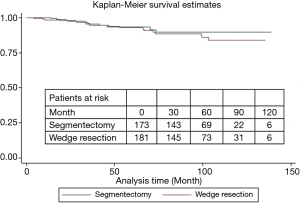
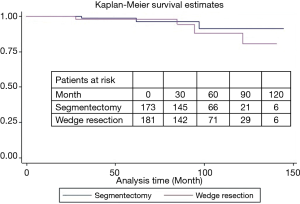
During a median follow-up of 78 months, there were 15 local recurrences: 7 (4.0%) in group I and 8 (4.4%) in group II, while no patient with GGO type tumors had local recurrence. There were 17 distant recurrences: 8 (4.6%) in group I and 9 (5.0%) in group II. Furthermore, overall and lung cancer-specific survivals were of no significant difference between the two groups. The 10-year overall survival rates were 89.5% in group I and 83.7% in group II, while the 10-year lung cancer-specific survival rates were 91.3% in group I and 88.1% in group II (Figures 1 and 2). In addition, lung cancer-specific survival of segmentectomy and wedge resection for patients with GGO type tumors was of no significant difference (100% vs. 100%). For patients with solid type tumors, 10-year lung cancer-specific survival rate after segmentectomy (83.1%) was higher than after wedge resection (69.0%), though the difference did not reach statistical significance (P=0.27) (Figure 3). Lung cancer -specific survival rates at 10 years were significantly better in patients with GGO type tumors than in those with solid type tumors (100% vs. 76.9%, P<0.001) (Figure 4). In multivariate Cox regression analyses of disease-free survival of all patients, GGO type was the only independent prognostic factor, while extent of resection did not have any influence on survival (Table 3).
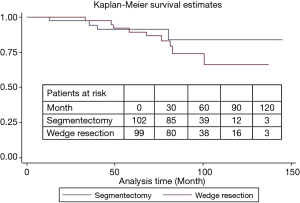
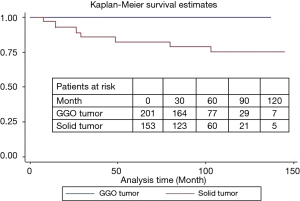
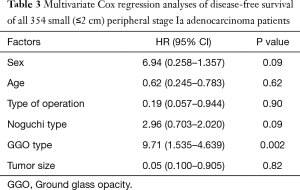
Full table
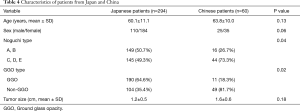
In this international study, 10-year overall survival of Japanese patients was higher than that of Chinese patients (93.8% vs. 61.1%, P=0.003, Figure 5). However, 10-year overall survivals of Japanese and Chinese patients with GGO type tumors receiving sublobar resection were of no significant differences (94.2% vs. 100%, P=0.78) (Figure 6). Characteristics of patients from two countries are summarized in Table 4. The percentages of Noguchi type A, B and GGO type tumor in Japanese patients were significantly higher than in Chinese patients (P=0.04 and 0.02, respectively).
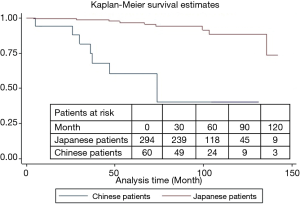
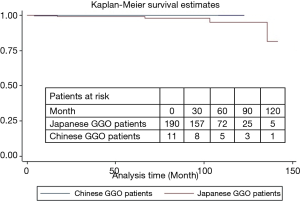
Full table
Discussion
In 1995, a randomized trial by LCSG (the Lung Cancer Study Group) demonstrated that sublobar resection for stage I NSCLC was oncologically inferior to lobectomy in terms of local recurrence and survival rate (1). Since then, lobectomy has been recommended as the gold standard treatment for stage I NSCLC, while sublobar resections was only reserved for patients who could not tolerate lobectomy due to marginal lung function and (or) significant comorbidities. However, tumors with diameter exceeding 2 cm were also included in the LCSG study, and radiologic or histologic subtypes of tumor were not mentioned. In recent years, an increasing number of small-sized (diameter ≤2.0 cm) tumor and ground glass nodules (GGNs) are detected because of increased use of CT screening (7,8). Hence, the mandatory use of lobectomy for such patients is now being questioned, while intentional sublobar resection is performed more frequently (8). Today, many single-institution retrospective studies have demonstrated that sublobar resection may provide comparable long-term results to lobectomy in selected small-sized (diameter ≤2.0 cm) stage Ia NSCLC patients (3-5).
Besides tumor size (9,10), CT appearance and corresponding histology subtypes of tumor were also reported to be associated with the outcomes of sublobar resection (11). In 1995 Noguchi et al. reviewed 236 surgically resected small (≤2 cm) peripheral adenocarcinomas and proposed a histologic classification of 6 types based on tumor growth patterns. According to the new IASLC proposal, AAH and AIS are mostly pGGO, corresponding to Noguchi types A and B, while MIA often appears as mixed GGO, roughly coincides with type C in Noguchi’s classification. GGO type tumors (proportion of GGO equal or more than 50% in HRCT) have minimal or no invasive growth, and are usually small in size (<3 cm). Sublobar resection is thus considered suitable for such tumors, and is even suggested as an better option for pGGO (11,12).
In this study, no patient with GGO type tumors had local recurrence, while Lung cancer -specific survival rates were significantly better in patients with GGO type tumors than in those with solid type tumors. In addition, our study also demonstrates that GGO type is an independent prognostic factor for disease-free survival in small-sized (diameter ≤2.0 cm) stage Ia adenocarcinomas. All these results support the notion that sublobar resection is acceptable for small GGO type adenocarcinomas.
Compared with anatomical segmentectomy, non-anatomical wedge resection was reported to be an inferior oncologic approach (9,13,14). Anatomical segmentectomy has the theoretical advantage of a more comprehensive resection, reduced technical limitations for achieving appropriate margins, and wider resection of draining lymphatics as a potential source of residual cancer cells (15). Miller et al. (13) compared clinical outcomes of segmentectomy and wedge resection for small-sized stage Ia lung cancer, and found there was statistically significant survival advantage and improved local control favoring segmentectomy even in very small tumors. Sienel et al. (7) also found significantly less local recurrence (55% vs. 16%) and improved cancer-specific survival (71% vs. 48%) after anatomic segmentectomy with systematic nodal dissection than after wedge resection with selective nodal sampling. However, for selected small-sized stage Ia adenocarcinomas, wedge resection may produce equivalent oncologic results when compared with segmentectomy. Okada et al. (6) revealed that wedge resections with lymph node sampling yield a disease-free and overall survival equivalent to segmentectomies and lobectomies in patients with stage Ia NSCLC ≤2 cm who could even tolerate a lobectomy. In our study, morbidity rate of wedge resection was lower than that of segmentectomy, and local recurrence rates were comparable between two groups. There was no significant difference in either overall survival or lung cancer-specific survival following two types of operation. Our results suggest that wedge resection may not be inferior to segmentectomy for selected small-sized (≤2 cm) stage Ia adenocarcinomas.
Increased local recurrence after wedge resection was attributed to the technical limitations of achieving an appropriate margin and incomplete dissection lymph nodes (14). During sublobar resection in this study, frozen-section analysis of sampled lobar, hilar, and mediastinal lymph nodes was mandatory, when conversion to lobectomy was indicated while node was proved to be positive. In addition, the LN metastases rate of small-sized lung cancer was reported to be as low as 0–5%. Therefore, intentional wedge resection may be accepted for a small peripheral T1N0 cancer, provided that sampling of N1 and N2 nodes was carried out during wedge resection. Several studies revealed that width of resection margins was an important factor to determine local recurrence following sublobar resection (16-18). Swanson (16) indicated that a safety margin of >20 mm might be reasonable, because resection margin <20 mm tended to be associated with higher rate of local recurrence. In this study, the parenchymal surgical margin for all sublobar resection was at least 2 cm, which can account for the low local recurrence rates of both groups.
An interesting finding need to point out is that the percentage of Noguchi type A, B and GGO type tumors in Japanese patients was higher than Chinese patients. Our results indicated that lung cancer patients receiving sublobar resection in Japan were more frequently at an earlier pathological type than those in China. One important reason is that CT screening is more popular in Japan, with more lung cancer patients being detected at an earlier stage. Accordingly, overall survival of Japanese patients was higher than that of Chinese patients, but overall survivals of Japanese and Chinese patients with GGO type tumors were of no significant difference.
This study has several some limitations. As a retrospective study, potential selection bias is inevitable. Second, the clinical results of lobectomy for such small-sized tumors in the same period were not recorded, thus comparison of lobectomy and sublobar resection cannot be achieved. Third, classification of adenocarcinoma recommended by the International Association for the Study of Lung Cancer (IASLC) in 2011 was not used in this retrospective study, as all patients were treated before 2012.
Despite these limitations, our results suggest that sublobar resection is acceptable for small adenocarcinomas without nodal involvement, and wedge resection may not be inferior to segmentectomy for a GGO type tumor 20 mm or less in diameter. Our study also demonstrates that GGO type is an independent prognostic factor for disease-free survival in small-sized (diameter ≤2.0 cm) stage Ia adenocarcinomas. Though we still regard lobectomy as the ‘‘gold standard’’ in surgical management of NSCLC, prospective study over a longer follow-up period is warranted to prove the efficacy of limited resections, especially wedge resection, for this special group of patients. Currently two prospective trials (JCOG0802 and CALGB140503) are ongoing to compare disease-free survivals of patients with small (≤2.0 cm) peripheral stage Ia NSCLC undergoing lobectomy or sublobar resection (8). However, oncological results of segmentectomy and wedge resection are not compared separately. What is more, the IASLC (the International Association for the Study of Lung Cancer) Classification of Lung Adenocarcinoma is not employed in either of these two trials. Therefore, prospective trials with the intention to compare the results of segmentectomy and wedge resection are definitely in need to clarify this issue.
In conclusion, sublobar resection is an acceptable procedure for small lung adenocarcinomas without nodal involvement, while wedge resection may not be inferior to segmentectomy for small GGO type tumors. GGO type is an independent prognostic factor of disease-free survival for small-sized (diameter ≤2.0 cm) stage Ia lung adenocarcinomas.
Acknowledgements
Funding: This work was supported by the Science and Technology Commission of Shanghai Municipality (No. 14411950800).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of the hospitals. Informed consent from patients was waived because of the retrospective nature of the study (Number/ID of the Ethic Approval is ks14025).
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Shapiro M, Weiser TS, Wisnivesky JP, et al. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancer. J Thorac Cardiovasc Surg 2009;137:1388-93. [Crossref] [PubMed]
- Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. [Crossref] [PubMed]
- Yamashita S, Chujo M, Kawano Y, et al. Clinical impact of segmentectomy compared with lobectomy under complete video-assisted thoracic surgery in the treatment of stage I non-small cell lung cancer. J Surg Res 2011;166:46-51. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Sienel W, Dango S, Kirschbaum A, et al. Sublobar resections in stage IA non-small cell lung cancer: segmentectomies result in significantly better cancer-related survival than wedge resections. Eur J Cardiothorac Surg 2008;33:728-34. [Crossref] [PubMed]
- Asamura H. Role of limited sublobar resection for early-stage lung cancer: steady progress. J Clin Oncol 2014;32:2403-4. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection J Thorac Cardiovasc Surg 2005;129:87-93. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. [Crossref] [PubMed]
- Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. [Crossref] [PubMed]
- Nakamura H, Saji H, Ogata A, et al. Lung cancer patients showing pure ground-glass opacity on computed tomography are good candidates for wedge resection. Lung Cancer 2004;44:61-8. [Crossref] [PubMed]
- Miller DL, Rowland CM, Deschamps C, et al. Surgical treatment of non-small cell lung cancer 1 cm or less in diameter. Ann Thorac Surg 2002;73:1545-50. [Crossref] [PubMed]
- Smith CB, Swanson SJ, Mhango G, et al. Survival after segmentectomy and wedge resection in stage I non-small-cell lung cancer. J Thorac Oncol 2013;8:73-8. [Crossref] [PubMed]
- Watanabe A, Ohori S, Nakashima S, et al. Feasibility of video assisted assisted thoracoscopic surgery segmentectomy for selected peripheral lung carcinomas. Eur J Cardiothorac Surg 2009;35:775-80. [Crossref] [PubMed]
- Swanson SJ. Video-assisted thoracic surgery segmentectomy: the future of surgery for lung cancer? Ann Thorac Surg 2010;89:S2096-S2097. [Crossref] [PubMed]
- Sienel W, Stremmel C, Kirschbaum A, et al. Frequency of local recurrence following segmentectomy of stage IA non-small cell lung cancer is influenced by segment localization and width of resection margins—implications for patient selection for segmentectomy. Eur J Cardiothorac Surg 2007;31:522-7. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62. [Crossref] [PubMed]


