Acute respiratory distress syndrome—a worldwide economic perspective
Introduction
The Lung Safe study (Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE) was an observational international study performed in 459 intensive care units (ICUs) worldwide during the winter 2014 in patients with hypoxemic acute respiratory failure (1). Among the large number of patients screened, 2,813 were meeting ARDS criteria according to the Berlin definition and analyzed (2). This is the largest study ever done in the field and actually size does matter. This secondary analysis explored the effects of geo-economic variations and their impact on practice and outcome in ARDS patients (3). The authors used the 2016 World Bank countries classification to define three major geo-economic grouping countries, namely European high-income (Europe-high), high-income in the rest of the world (rWORLD-High) and middle-income (Middle). Outcomes were compared across these three groups. We will first present the major results of this study, then discuss their significance and how these may impact clinicians for care delivery in patients with ARDS.
Results of the study
Patients’ characteristics
Of the 2,813 ARDS patients 1,521 (54%) were recruited from Europe-High, 746 (27%) from rWORLD-High, and 546 (19%) from Middle countries. Of note, no patient was enrolled from low-income countries, which probably reflects the limited availability of ICUs in resource-limited settings (4).
Although the patients from the three grouping regions significantly differed on their demographics, both the size and the effects of the difference on outcomes were small. Severity of ARDS varied by regions, with rWORLD-High countries having significantly more patients in the mild category and less patients in the severe category, and more patients with a mean partial pressure of arterial oxygen (PaO2) to fractional concentration of oxygen in air (FiO2) ratio lower than 150 mmHg.
Clinician recognition of ARDS was significantly less common in rWORLD-High than in Europe-High or Middle countries.
Ventilator management
There were striking differences in the management of patients with ARDS across regions, both in terms of ventilation strategies and adjunct treatments (Figure 1). Most patients received a tidal volume of 6 to 8 mL per kilogram predicted body weight (mL/kg) in all the three region groupings, but significantly fewer patients received lower tidal volumes in Middle countries, and more patients received tidal volumes higher than 10 mL/kg in Europe-High countries. FiO2 >0.6 was used more frequently in patients from Europe. Non-invasive ventilation was used in the same proportion of patients across groupings, but less frequently in more severe patients (those with a PaO2/FiO2 ratio <150 mmHg) from Middle countries. It could be that the difference in use of protective ventilation strategy and adjunct treatments shown in Figure 1 across geo-economic groupings follow cultural differences. Lung protective ventilation has been introduced in the US with the landmark ARMA trial and is more frequently used in r-WORLD-High. Neuromuscular blockade agents and prone position have been extensively studied in Europe and it is not really surprising that these interventions are more frequently used there.
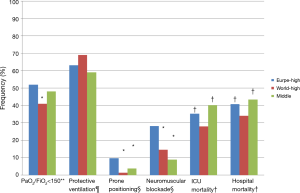
Both neuromuscular blockade and prone positioning were used more frequently in Europe-High countries. However, as pointed out by the authors, even in those countries, prone positioning was used only in less than 10% of patients with a PaO2/FiO2 ratio <150 mmHg, a population in which it has been demonstrated to improve survival (5).
Extra-corporeal membrane oxygenation (ECMO) was used in almost 11% of cases in Europe-High and rWORLD-High, but never in Middle countries.
Recruitment maneuvers were much more frequent in Europe-High and Middle countries (27.4% and 35.2% respectively) than in rWORLD-High countries (8.4%). After adjustment for ARDS severity, the regional differences in the use of adjunctive measures were not significant.
Patient outcome
There were major differences in outcomes across the 3 grouping regions (Figure 1). First, in patients from Europe-High countries mechanical ventilation (MV) and ICU stay were longer than those from rWORLD countries, a difference that persisted after adjustment for disease severity and other covariates. Similarly, the probability to be successfully weaned from MV was significantly greater in patients from rWORLD-High countries than in those from Europe-high or Middle countries. These findings could be explained by differences in both ventilation strategies and weaning practices.
The authors then used mixed-effect logistic regression in a two-level random intercept model to assess the association between patient-centered variables (first level), ICU-centered variables (second level) and outcomes (most importantly ICU and hospital mortality). Demographic, risk, illness severity, and management factors were included in the first level; gross national income per person, geographical area, and ICU characteristics (number of beds, academic or non-academic ICU, average number of nurses and physicians per patient, etc.) were included in the second level. The authors found that differences in approach to NV did not result in significant geo-economic variations regarding ICU or hospital mortality. Patient-level variables associated with hospital mortality included age, active or hematological neoplasm, chronic liver failure, acidosis, PaO2/FiO2 ratio, non-pulmonary sequential organ-failure assessment (SOFA) score, and respiratory rate. Interestingly, there was no significant association between ICU-level variables and hospital mortality.
One of the major findings of the study is that unadjusted ICU and hospital mortality were both significantly lower in rWORLD-High countries than in Europe-High or Middle countries. Most importantly, these differences persisted after adjustment for patient-level variables independently associated with survival: adjusted odds-ratio of hospital mortality were 0.32 [95% confidence interval (95% CI): 0.16–0.62] in rWORLD-High countries, and 0.51 (0.26–0.998) in Europe-High countries when compared to Middle countries. Adjusted odds-ratio of ICU-mortality were 0.37 (0.26–0.52) in rWORLD-High countries, and 0.47 (0.43–0.77) in Europe-High countries when compared to Middle countries.
Finally, in a separate multivariate analysis across all the countries (with no region grouping factor used) the mortality in ARDS from any cause was independently associated with gross domestic product: patients from the Middle countries had the lowest averaged probability of survival from ARDS, a result that is in line with the mortality difference observed across grouping regions. This provocative finding adds to the growing body of evidence showing that there is an association between socioeconomic status and survival from illnesses (5). Therefore, the LUNG SAFE is the first to show up this association in the specific context of ARDS.
Discussion of the results
This study is the first to identify differences in the disease severity, clinical recognition, treatment modalities and outcomes of ARDS patients between geo-economic groups of countries based on gross national income per person. One original finding of this study is the independent association of gross domestic product with all-cause mortality in ARDS patients. Interestingly, this association was not explained by technical or human resources of the participating ICUs; the number of beds, the percentage of ICU beds per hospital, the number of beds per nurse and per physician were not associated with all-cause hospital mortality. Hospital and ICU mortality were significantly higher in middle-income countries compared to high-income countries. While other factors—discussed below—may contribute to this difference in survival, one may wonder how socio-economic status affects the outcome of critical illness. In 2008, the World’s Health Organization has published a report on the social determinants which detailed the role played by social gradient, stress, early life events, social exclusion, work, social support, addiction, food and transport in affecting health and life expectancy (6). In a multicenter retrospective study of more than 33,000 patients admitted to the ICU, the presence of a compensable insurance status was independently associated with a reduction in mortality (7). In another study conducted in Germany, low socio-economic status (evaluated by patient’s education and professional qualification, occupational position and net household income) was associated with higher disease severity and length of stay in ICU (8). In a large international prospective study of more than 10,000 ICU patients, in-hospital death was associated with lower global national income (5). A recent study conducted in Denmark on ICU septic patients, low income was significantly associated with increased 30-day mortality (9). Similar association between socio-economic status and outcome was found in non-critically-ill patients with asthma (10), breast cancer (11) and ischemic heart disease (12). Genetic susceptibility (and therefore ethnic heterogeneity) may also contribute to mortality in ARDS patients. While most genetic studies have focused on ARDS pathogenesis, some have linked genetic variants and polymorphisms with patient outcome (13). However, the three groups in this study have been defined according to economic criteria, and except for the high-income European group, the remaining 2 groups include countries from different continents and racial backgrounds. The link between gross domestic product and mortality may reflect better access to medical services and more effective preventive healthcare policies. One of the limits of this study is not including low-income countries, which might have confirmed the influence of geo-economic variations on patient outcome in ARDS.
As mentioned above, other factors may explain the mortality difference between the 3 geo-economic groups. Several baseline characteristics were different between the 3 groups and were associated with all-cause hospital mortality. For example, compared to patients from the rest of world high-income countries, patients from European high-income countries were slightly older, had more severe ARDS and lower PaO2/FiO2 ratio. However, patients from European high-income countries had more chronic liver failure. Precise causes of mortality were not detailed but respiratory failure was the leading cause of death (40%), which is surprisingly high with respect to the percentage of severe ARDS (18% to 25%). One of the limits of the mortality analysis is the fact that patients who were discharged from the hospital before day 28 were considered alive and that inpatients still hospitalized at day 90 were also considered to have survived till discharge. ICU mortality at day 90 varied from 28% to 40% and hospital mortality at day 90 varied from 34% to 43%. Mortality varied according to the disease severity. This high mortality rate is similar to what is published in ARDS studies (14-16), but yet higher than the 23% and 31% mortality rate at day 90 in the treatment arms of the PROSEVA (17) and ACURASYS (18) trials evaluating prone positioning and neuromuscular blockers, respectively. It should be mentioned that the mortality rate in the general population is different across countries. As an example, the US age-adjusted mortality rate amounted to 7.2/1,000 inhabitants in 2014 (19). In 2015 in Europe this rate was 10.2/1,000 (20). The ICU mortality rate observed in the Lung Safe study for ARDS patients essentially follows the general mortality rate in the corresponding geo-economic groupings.
This finding leads us to discuss another interesting though alarming result of this study, namely the ARDS management regarding three proven treatment measures in ARDS i.e., protective ventilation (21), prone positioning (17) and neuromuscular blockade (18). Although there is still an area of uncertainty for the latter, experts recently recommended lower tidal volume for ARDS and prone positioning for the severe category (22). Overall, only 63% of intubated patients received protective ventilation (with no difference between geo-economic groups), while less than 10% received prone positioning (contrasting with 41% to 52% of patients with a PaO2/FiO2 <150 mmHg), and 5% to 20% of patients had neuromuscular blocking agents, with variability among the 3 groups. Plateau pressures were >30 cmH2O in 10% of patients. The variations in the use of adjunctive measures were not explained by differences in severity profile or by difference in recognition of ARDS. The authors suggest that the more frequent use of prone positioning and neuromuscular blockade in European high-income countries may reflect the fact that these techniques have been developed in Europe and might not have fully integrated routine care in non-European countries. However, one can argue that the current possibilities to access to medical information and to share it in the international medical community should overwhelm this barrier. The treatment measures that have proven benefit to ARDS patient outcome should be implemented more efficiently in the daily practice. The high financial cost of extracorporeal membrane oxygenation may explain its low rate of use in middle-income countries. However, plateau pressure monitoring, prone positioning and neuromuscular blockade agents are less expansive. Moreover, prone positioning requires a practical expertise that may be lacking in the ICUs. Understanding the reasons why intensivists in this study, who were mostly from academic ICUs, did not use these treatments to a larger extent constitutes the first step to address this problem. A recent international prevalence study about the use of prone position in ARDS patients found that 32.9% of those in the severe category received the intervention, a 2-fold higher rate than in the Lung Safe study (23). Furthermore, in this study the primary reason for not proning was that hypoxemia was not judged severe enough by the clinicians (23). In an editorial about the LUNG SAFE study and the lack of use of evidence-based therapy, Parsons et al. elegantly compared this situation to a philosophical dilemma by asking: “if a tree falls in a forest and there is no one in the woods to hear it, does it make a sound?” (24).
A similarly worrying result in this study is the under-diagnosis of ARDS. Clinicians recognized ARDS in 62% of included patients, with a higher rate for more severe disease. Optimal treatment of ARDS requires optimal diagnosis. No clear explanation is available to explain this finding especially that 65% of participating ICUs were in academic hospitals. On the other hand, clinical recognition was assessed in comparison to a computer algorithm using the raw data that make up the components of the Berlin definition. The diagnostic accuracy of this algorithm was not validated, especially for the differential diagnosis of bilateral radiological infiltrates and hypoxemia.
The large size of this international multicenter cohort is a major strength of this study, along with the provocative picture that it gives us about the (under) treatment of ARDS. The predominance of academic ICUs and the absence of data from low-income countries are selection bias which may hinder the cohort’s representativeness.
Conclusions
This study has confirmed that clinical presentation of ARDS and patterns of care varied significantly across geo-economic groups of countries defined by gross national income per person. It describes for the first time an association between gross domestic product and hospital mortality in ARDS. It also highlights the big gap between “theoretical” evidence-based medicine and clinical daily practice. This study should be the starting point for further studies to understand the reasons for the limited use of proven therapeutic strategies in ARDS patients and address them, in order to improve patient outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Laffey JG, Madotto F, Bellani G, et al. Geo-economic variations in epidemiology, patterns of care, and outcomes in patients with acute respiratory distress syndrome: insights from the LUNG SAFE prospective cohort study. Lancet Respir Med 2017;5:627-38. [Crossref] [PubMed]
- Dondorp AM. IIyer SS, Schultz MJ. Critical Care in Resource-Restricted Settings. JAMA 2016;315:753-4. [Crossref] [PubMed]
- Vincent JL. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med 2014;2:380-6. [Crossref] [PubMed]
- World Health Organization. Closing the gap in a generation: health equity through action on the social determinants of health. Final Report of the Commission on Social Determinants of Health. 2008.
- Gabriel LE, Bailey MJ, Bellomo R, et al. Insurance status and mortality in critically ill patients. Crit Care Resusc 2016;18:43-9. [PubMed]
- Bein T, Hackner K, Zou T, et al. Socioeconomic status, severity of disease and level of family members' care in adult surgical intensive care patients: the prospective ECSSTASI study. Intensive Care Med 2012;38:612-9. [Crossref] [PubMed]
- Schnegelsberg A, Mackenhauer J, Nibro HL, et al. Impact of socioeconomic status on mortality and unplanned readmission in septic intensive care unit patients. Acta Anaesthesiol Scand 2016;60:465-75. [Crossref] [PubMed]
- To T, Simatovic J, Zhu J, et al. Asthma deaths in a large provincial health system. A 10-year population-based study. Ann Am Thorac Soc 2014;11:1210-7. [Crossref] [PubMed]
- Nattinger AB, Wozniak EM, McGinley EL, et al. Socioeconomic Disparities in Mortality Among Women With Incident Breast Cancer Before and After Implementation of Medicare Part D. Med Care 2017;55:463-9. [Crossref] [PubMed]
- Cafagna G, Seghieri C. Educational level and 30-day outcomes after hospitalization for acute myocardial infarction in Italy. BMC Health Serv Res 2017;17:18. [Crossref] [PubMed]
- Flores C, Pino-Yanes MM, Casula M, et al. Genetics of acute lung injury: past, present and future. Minerva Anestesiol 2010;76:860-4. [PubMed]
- Linko R, Okkonen M, Pettila V, et al. Acute respiratory failure in intensive care units. FINNALI: a prospective cohort study. Intensive Care Med 2009;35:1352-61. [Crossref] [PubMed]
- Li G, Malinchoc M, Cartin-Ceba R, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med 2011;183:59-66. [Crossref] [PubMed]
- Villar J, Blanco J, Anon JM, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 2011;37:1932-41. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Papazian L, Forel J, Gacouin A, et al. ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: Final Data for 2014. Natl Vital Stat Rep 2016;65:1-122. [PubMed]
- Eurostat. 2017. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Mortality_and_life_expectancy_statistics
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Fan E, Del Sorbo L, Goligher EC, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017;195:1253-63. [Crossref] [PubMed]
- Guérin C, Beuret P, Constantin JM, et al. A prospective international observational one-day prevalence study on prone positioning in ARDS. The APRONET study. Intensive Care Med 2018;44:22. [Crossref] [PubMed]
- Parsons PE, Stapleton RD. Geo-economic Variations and ARDS: If a Tree Falls in a Forest. Chest 2017;152:461-2. [Crossref] [PubMed]


