Characteristics of endobronchial tuberculosis patients with negative sputum acid-fast bacillus
Introduction
Endobronchial tuberculosis (EBTB) is defined as a tuberculous infection of the tracheobronchial tree with microbial and histopathological evidence, with or without parenchymal involvement (1,2). EBTB was first shown in 1698 by Richard Morton in an autopsy case died due to tuberculosis (3). The pathogenesis of EBTB is not well understood. Nevertheless five potential mechanisms was believed to be responsible from the development of endobronchial infections caused by M. tuberculosis: (I) direct invasion from an adjacent paranchymal focus; (II) implantation of the organisms from infected sputum; (III) haematogenous spread; (IV) erosion of a lymph node inside a bronchus; (V) lymphatic drainage from the parenchyma towards the peribronchial region (4-7). Since bronchoscopy is not routinely performed to all patients with pulmonary tuberculosis, actual incidence of EBTB could not be evaluated. For this reason, the proportion of endobronchial involvement in active pulmonary tuberculosis is variable according to the literature. In a study it is reported that 5.88% of pulmonary tuberculosis cases were shown to have EBTB (4); in another study this ratio was reported to be 10-40% (7) and in other two studies this ratio was shown to be 50% (8,9). EBTB may mimic diseases like bronchial asthma, pneumonia and lung cancer (10,11). EBTB may affect any region of the tracheobronchial tree. If it affects the middle lob, it causes collapse, since the entry of the middle lob is narrow. This is known as middle lob syndrome (12,13). Chung classified forms of EBTB into seven subtypes by bronchoscopic finding: actively caseating, edematous-hyperemic, fibrostenotic, tumorous, granular, ulcerative, and nonspecific bronchitic (14). EBTB is a severe situation with high bacilli lode, and may cause complications with high morbidity like bronchial stenosis; early diagnosis and treatment is therefore mandatory (1,4,9). The course and prognosis of endobronchial TB are variable, ranging from complete clearance to severe bronchostenosis (15). In this study, clinical, radiological and bronchoscopic characteristics of cases diagnosed to have EBTB were evaluated.
Patients and methods
One hundred seventy eight patients with active lung tuberculosis (128 AFB positive, 50 ARB negative) attending to Yedikule Chest Diseases and Thoracic Surgery Training and Research Hospital between 2008 and 2012 were examined. Twenty of 50 AFB (-) patients were given anti-tuberculous therapy as clinical and radiological. Thirty of 50 AFB (-) patients were performed fiberoptic bronchoscopy (FOB) and determined endobronchial lesion in 16 patients. Fourteen patients with normal FOB were similar to 16 patients with endobronchial lesion as characteristic of clinical and radiological. These 16 EBTB patients with at least three negative sputum examinations for AFB and diagnosed as having EBTB on the histopathological examination of bronchoscopically obtained specimens showing granulomatous structures with caseation necrosis and/or positive AFB-culture on the microbiological examination of bronchoscopically obtained specimens were included in the study. Our study was a retrospective observational study. This study was performed according to the Helsinki declaration 2008 and written informed consents were obtained from all patients. Age, sex, symptoms, tuberculin skin test (TST) results, microbiological examination results and radiological findings on postero-anterior chest X-rays of the cases were recorded. Computerized tomography (CT) findings of the cases were revised. Bronchoscopical lesions were classified according to Chung classification (14). Actively caseating lesions were grouped as type 1, oedematous hyperemic lesions as type 2, fibrostenotic lesions as type 3, tumorous lesions as type 4, granular lesions as type 5, ulcerative lesions as type 6 and non-specific bronchitic lesions as type 7. After diagnosis, cases were treated under direct observation with 5 mg/kg·day izoniacid, 10 mg/kg·day rifampicin, 20 mg/kg·day ethambutol and 25 mg/kg·day pyrazinamid. Response to treatment were checked with chest X-ray and thorax CT examinations. After a two month therapy patients becoming AFB culture negative and not showing any resistance to antituberculous therapy were continued to be treated with two drug combination. Antituberculous treatment was stopped after a 6-month treatment. Bronchoscopy was repeated in patients with delayed recovery and treatment was continued for nine months. Systemic corticosteroid treatment was added to patients with bronchial stenosis.
Results
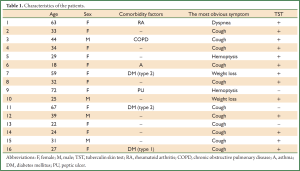
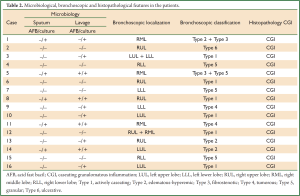
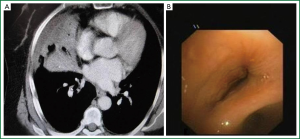
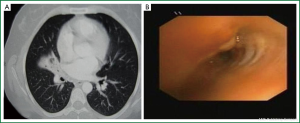
Age, sex, comorbidity factors, symptoms and TST were given in Table 1. EBTB was found to be more common in females (M/F ratio: 4/12=1/3), prior bronchoscopy cases were at least three times examined for sputum AFB (with Ziehl-Neelsen stain) and all were AFB negative. In 4 of the cases bronchoscopic lavage AFB were positive, in other 10 cases AFB cultures were positive (Table 2). Most common symptoms were cough (16 cases, 100%), sputum (12 cases, 75%), weight loss (10 cases, 62.5%), hemoptisis (6 cases, 37.5%), chest pain (4 cases, 25%) and dyspnea (2 cases, 12.5%) respectively. Radiological examination findings revealed consolidations/infiltrations (14 cases, 87.5%), nodular lesions (6 cases, 37.5%), cavitary lesions (4 cases, 25%) and unilateral (7 cases, 43.7%) or bilateral hilar widening (5 cases, 31.2%) and atelectasia (4 cases, 25%). Middle lob syndrome because of collapse was seen in 3 cases (Figures 1,2). Most common lesions observed bronchoscopically were active caseous lesions (Type 1, in 6 cases, 37.5%). Granular lesions (Type 5) were seen in 3 cases (18.7%), tumorous lesions (Type 4) in 2 cases (12.5%), edematous hyperemic lesions (Type 2) in 2 cases (12.5%), edematous hyperemic (Type 2) + fibrostenotic (Type 3) lesions were seen in 1 case (6.2%), granular (Type 5) + fibrostenotic (Type 3) lesions (6.2%) were seen in 1 case (Table 2, Figures 1-4). In all cases “granulomatous inflammation showing caseation” was shown in the histopathological examination of biopsy specimens. Lesions were totally recovered in 14 patients treated with standard anti-tuberculous treatment. Only two patients with middle lob syndrome (edematous – hyperemic + fibrostenotic and granular + fibrostenotic) needed treatment longer than six months for complete recovery, they were treated for nine months and systemic corticosteroids were added because of stenotic appearance; partial response were observed.
Full table
Full table
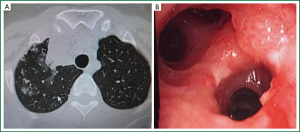
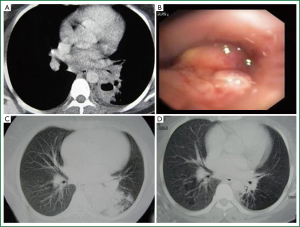
Discussion
EBTB contains rather high amounts of tuberculosis bacilli. Early diagnosis and treatment is important for prevention of the spread of tuberculosis and complications like cicatricial bronchostenosis due to endobronchial involvement (16). More than half of the EBTB cases were reported to be younger than 35 years. Different female/male ratios were reported. It was shown that it is more common in young woman than male sex (4,15,17). It was more common in young woman also in our study with a male/female ratio (M/F: 1/3). However in a recent study it was also common in elderly (8); sites of tracheabronchial invasion were reported to be different in young and elderly patients (18). In elderly patients lobar and segmental bronchial invasion was more common, whereas in younger patients involvement of trachea and main bronchi were seen generally and middle lobe syndrome was more common in elderly (19). In our study all of the three cases of middle lobe syndrome were females and their ages were 29, 63 and 67.
Classical symptoms of EBTB are cough, difficultly expectorated high viscose sputum, wheezing, fever, chest pain and haemoptysis (16). These symptoms may be seen in other respiratory diseases, so they do not help for the early diagnosis of EBTB. The most common symptom is persistent cough, which is thought to be related to the endobronchial inflammation (16). In a study the most common symptoms reported were cough, sputum, dyspnea and fever (20). In our study most common symptoms were cough and difficult sputum expectoration followed by weight loss, haemoptysis, chest pain and dyspnea in frequency.
Microbiological examination of sputum is the first coming main examination leading to diagnosis in EBTB. Nevertheless unlike paranchymal diseases AFB positivity in EBTB is between 16% to 53.3% in most favorable conditions. In a study performed by Lee et al. sputum ARB positivity prior bronchoscopy was found to be as low as 17% (21). This ratio increased to 73.6% with culture (7). Sputum AFB was negative in all of our cases at the beginning of the study. In our study, bronchoscopic lavage revealed positive AFB in 4 cases and positive culture in 10 cases.
Radiological findings of EBTB may vary, different findings like patchy alveolar infiltrations, atelectasia, hilar widening, pleural effusion, mass and cavitary lesions may be seen (16). EBTB can not be excluded in the case with normal chest X-ray, because 10% to 20% of cases may have normal chest X-ray (16). In the study conducted by Lee et al. it was reported that common radiological findings of EBTB were consolidations and volume loss, and these findings were seen in 83.4% of the cases (21). Chest X-ray frequently showed atelectasis. This would make it difficult to differentiate EBTB not only from bronchial asthma, but also from bronchogenic carcinoma in old age (21). Thorax CT is a helpful tool to show endobronchial masses, widened mediastinal-hilar lymph nodes, helps with better identification of paranchymal lesions and evaluation of cases like stenosis or obstruction (2,16). In a study multiple lobar lesions, exudative shadows and atelectasis were the most common radiological findings (20). Radiological findings of our cases listed according to their frequency were consolidations/infiltrations, nodular lesions, cavitary lesions and uni- or bilateral hilar widening.
Bronchoscopy should be performed in suspicious cases like unexplained cough, wheezing, dyspnea or haemoptysis (22). Persistent segmental or lober collaps, lobar infiltrations and obstructive pneumonia findings on chest X-ray examination are also indications for bronchoscopy (7). In a study common right upper lobe and right main bronchial EBTB were diagnosed with bronchoscopy (21). In our study, common bronchoscopic localization were right upper lobe (RUL), left upper lober (LUL) and right middle lobe (RML).
In a study active caseous type was reported to be most common (17). In recent studies it was reported that most common bronchoscopic finding was edematous-hyperemic type (8,23). This is the most common type of bronchoscopic finding in middle lobe syndrome (19). Sputum or bronchial lavage AFB is generally positive in active caseous type, but edematous type is hard to diagnose, and sputum and bronchial lavage AFB is generally negative; therefore tuberculosis culture and histopathological examinations should be performed (19). It is also reported that while active caseous type recovers almost completely, edematous or active caseous + edematous type changes to fibrostenotic type in more than 60% of cases (4). Four of the subtypes—actively caseating, edematous-hyperemic, fibrostenotic, and tumorous EBTB—show varying degrees of luminal narrowing of the bronchus, while the other three subtypes—granular, ulcerative, and nonspecific bronchitic EBTB—do not (4). Similarly, it is reported in a study that early stage exudative, granular and ulcerative lesions recovered without sequel; caseous and tumorous lesions of advanced disease might lead complications such as bronchostenosis causing bronchectasia (16). A study conducted by Um SW et al. revealed that factors related to development of bronchial stenosis were age over 45 years, pure or combined fibrostenotic subtype and symptoms lasting more than 90 days prior treatment (15). In our study most common lesions observed bronchoscopically were active caseous lesions. These were followed by granular, edematous-hyperemic, tumorous, fibrostenotic and ulcerative lesions with decreasing frequencies. Treatment responses of our cases were followed by control thorax CTs. There are literature reporting that follow up with CT might be an alternative to follow up with bronchoscopy (16). There are also some reports proposing that findings related to a development of bronchial stenosis could also be shown with CT examination (24). In our study, two middle lobe cases without a complete recovery after treatment, undergone control bronchoscopy in addition to control CT examinations and the findings were concordant with control CTs. As reported in other studies mentioned above pure or combined fibrostenotic types were found to be responding slower to treatment as seen in the two cases in our study.
Corticosteroids have been used empirically in the treatment of tuberculosis in an attempt to prevent fibrosis. However, the value of using corticosteroids for EBTB is uncertain (25). Though literature reporting that steroid addition did not help with improvement or clinical healing (5,6,26) there are literature arguing that oral or inhalated steroids effect improvement and clinical healing positively in some types of EBTB (2,27,28). Corticosteroids are likely to be beneficial in earlier stages when hypersensitivity is the predominant mechanism, but are unlikely to be helpful in more advanced cases when extensive fibrosis is present. Close follow-up is advisable as stenosis may develop later despite antituberculosis chemotherapy with or without corticosteroids (21). Except the two cases all of our cases were healed totally with standard antituberculous treatment (quaternary treatment for the first two months, binary treatment for the following four months, totally six months treatment). There were delayed response to treatment due to bronchostenosis in two of our cases (a case of edematous hyperemic + fibrostenotic type and a case with granular + fibrostenotic lesions). In these cases antituberculous treatment were continued for nine months and oral corticosteroids were added, however partial response to stenosis were obtained. This may support the findings reported in some literature mentioned above that corticosteroids do not help much with improvement in the treatment of bronchial stenosis.
Conclusions
EBTB can cause various radiological and bronchoscopical findings. In most of the cases distinct response is seen to antituberculous treatment. Bronchial stenosis is an important complication and should not be disregarded. Patients with old age, having symptoms lasting for a long time prior treatment and having fibrostenotic type of disease are at higher risk. Treatment should be given as soon as possible to avoid this complication.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hoheisel G, Chan B., Chan C, et al. Endobronchial tuberculosis: diagnostic features and therapeutic outcome. Respir Med 1994;88:593-7. [PubMed]
- Rikimaru T. Endobronchial tuberculosis. Expert Rev Anti Infect Ther 2004;2:245-51. [PubMed]
- Hudson EH. Respiratory tuberculosis: Clinical diagnosis. In: Heaf ERG. eds. Symposium on Tuberculosis. London: Cassell and Co., 1957:321-464.
- Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest 2000;117:385-92. [PubMed]
- Starke JR, Munoz F. Tuberculosis. In: Kliegman RM, Stanton B, St Geme J, et al. eds. Nelson Textbook of Pediatrics (16th ed). Philadelphia: W.B. Saunders Co., 2000:885-97.
- Starke JR. Tuberculosis. In: Katz SL, Hotez PJ, Gershon AA, et al. eds. Krugman’s Infectious Diseases of Children (10th ed). St. Louis: Mosby-Year Book Inc., 1998:571-604.
- Kashyap S, Mohapatra PR, Saini V. Endobronchial tuberculosis. Indian J Chest Dis Allied Sci 2003;45:247-56. [PubMed]
- An JY, Lee JE, Park HW, et al. Clinical and bronchoscopic features in endobronchial tuberculosis. Tuberc Respir Dis 2006;60:532-9.
- Kurasawa T, Kuze F, Kawai M, et al. Diagnosis and management of endobronchial tuberculosis. Intern Med 1992;31:593-8. [PubMed]
- Williams DJ, York EL, Nobert EJ, et al. Endobronchial tuberculosis presenting as asthma. Chest 1988;93:836-8. [PubMed]
- Matthews JI, Matarese SL, Carpenter JL. Endobronchial tuberculosis simulating lung cancer. Chest 1984;86:642-4. [PubMed]
- Gupta PP, Gupta KB, Agarwal D. Middle lobe syndrome due to tuberculous etiology: a series of 12 cases. Indian J Tuberc 2006;53:104-8.
- Gudmundsson G, Gross TJ. Middle lobe syndrome. Am Fam Physician 1996;53:2547-50. [PubMed]
- Chung HS, Lee JH, Han SK, et al. Classification of endobronchial tuberculosis by the bronchoscopic features. Tuberc Respir Dis 1991;38:108-15.
- Um SW, Yoon YS, Lee SM, et al. Predictors of persistent airway stenosis in patients with endobronchial tuberculosis. Int J Tuberc Lung Dis 2008;12:57-62. [PubMed]
- Yanardag H, Tetikkurt C, Tetikkurt S, et al. Computed tomography and bronchoscopy in endobronchial tuberculosis. Can Respir J 2003;10:445-8. [PubMed]
- Park EJ, Kim MO, Yang SC, et al. Clinical and bronchoscopic features of 280 patients with endobronchial tuberculosis (1990-2001). Korean J Med 2003;64:284-292.
- Kim HJ, Kim HS, Ma JE, et al. Clinical characteristics of endobronchial tuberculosis that develops in patients over 70 years of age. Tuberc Respir Dis 2007;63:412-16.
- Kim HC, Kim HS, Lee SJ, et al. Endobronchial tuberculosis presenting as right middle lobe syndrome: clinical characteristics and bronchoscopic findings in 22 cases. Yonsei Med J 2008;49:615-9. [PubMed]
- Qingliang X, Jianxin W. Investigation of endobronchial tuberculosis diagnoses in 22 cases. Eur J Med Res 2010;15:309-13. [PubMed]
- Lee JH, Park SS, Lee DH, et al. Endobronchial tuberculosis. Clinical and bronchoscopic features in 121 cases. Chest 1992;102:990-4. [PubMed]
- Teo SK. Endobronchial tuberculosis--a report of 5 cases. Singapore Med J 1990;31:447-50. [PubMed]
- Morrone N, Abe NS. Bronchoscopic findings in patients with pulmonary tuberculosis. J Bronchol 2007;14:15-8.
- Lee JH, Chung HS. Bronchoscopic, radiologic and pulmonary function evaluation of endobronchial tuberculosis. Respirology 2000;5:411-7. [PubMed]
- Chan HS, Sun A, Hoheisel GB. Endobronchial tuberculosis--is corticosteroid treatment useful? A report of 8 cases and review of the literature. Postgrad Med J 1990;66:822-6. [PubMed]
- Park IW, Choi BW, Hue SH. Prospective study of corticosteroid as an adjunct in the treatment of endobronchial tuberculosis in adults. Respirology 1997;2:275-81. [PubMed]
- Efferen LS. Tuberculosis: Practical solutions to meet the challenge. J Respir Dis 1999;20:772-85.
- García-Gasalla M, Yebra-Bango M, Villarreal García-Lomas M, et al. Resolution of symptoms of esophageal compression due to mediastinal tuberculosis after treatment with corticosteroids. Clin Infect Dis 1998;27:234. [PubMed]


