Expression of NF-κB, CD68 and CD105 in carotid atherosclerotic plaque
Introduction
One critical cause of strokes is embolism originating from unstable carotid plaques (1). Carotid atherosclerotic plaques develop from chronic lipid deposition and subsequent inflammation in carotid artery (2). Intraplaque neovascularization and vasa vasorum proliferation contribute in the progression or even rupture of atherosclerotic lesions (3). The vulnerability of atherosclerotic plaque is associated with plaque rupture, subsequently thrombus formation, embolization, and the cerebral vascular events are superimposed (1,4). Severity of carotid stenosis is associated with clinical manifestation (4). A variety of genes are highly expressed in human symptomatic plaques compared with asymptomatic ones, including insulin growth factor binding proteins (5), matrix metalloproteinase (MMP)-9, MMP-7, CD68 (1,6,7), vascular endothelial growth factor (VEGF) (8), lysosomal cathepsin, p53 (9), hepatocyte growth factor (10), and placenta growth factor (11). However, no definitive factors have been found to trigger the rupture of carotid plagues, and no effective way is available to predict the destabilization of carotid plaque in order to reduce the risk of stroke.
Atherosclerosis is associated with chronic inflammation of the vascular wall. CD68 protein is a pan macrophage marker and involves in inflammation of carotid plaque. The macrophage plays an important role in this inflammation. And it is reported that the degree of infiltrated macrophage and mast cell and microvascular density in the plaque is helpful in predicting the risk of rupture of vulnerable carotid plaques in both symptomatic and asymptomatic patients (7,12,13).
Rel or nuclear factor-kappa κ (NF-κB) proteins are composed of a group of structurally-related eukaryotic transcription factors, they include five NF-κB proteins in mammals: RelA/NFκB-p65, RelB, c-Rel, NF-B1/NFκB-p105, and NF-B2/NFκB-p100. These factors regulate normal cellular and organismal processes, and some abnormal conditions. After activation by various stimuli, NF-κB translocates into the nucleus and stimulates the expression of genes involved in several biological functions. Inappropriate activation of NF-κB has been associated with many inflammatory diseases while persistent inhibition of NF-κB leads to inappropriate immune cell development or delayed cell growth.
NF-κB plays a pivotal role in atherosclerotic plaques and peripheral blood mononuclear cells in patients with carotid atherosclerotic stenosis (14). The expression of NF-κB is not detectable in normal carotid artery of animals, but can be detected after balloon-induced injury of the carotid artery (15). More plaques with large atheroma and heavy plaque calcifications have developed gradually as the patients with risk factors of atherosclerosis get aged.
CD105 protein is related with development of new vessels. The macrophage and revascularization are associated with the progress of atherosclerotic plaque. Increased visualization and inflammation are considered as an important triggering factor to plaque vulnerability (16), whereas calcification is suggested to confer stability (17). Because we are not clear which factors contributing to the vulnerability of carotid plaque, development of carotid plaque involves the inflammatory process, and we hypotheses that NF-κB, CD68 and CD105 are highly expressed in carotid plaque, thus we investigated the expression of these factors in order to study the association of the plaque inflammatory mediators CD68, CD105 and NF-κB with patients’ clinical parameters of plaque instability.
Material and methods
Clinical characteristics of patients
A total of 43 patients (30 males and 13 females) with carotid stenosis from March 2008 to March 2011 were treated with selective carotid endarterectomy (CEA). Patients with carotid stenosis were grouped asymptomatic if they had not experienced any clinically apparent ischemic events such as transient ischemic attack (TIA) or stroke within the previous six months; otherwise, those with history of TIA or stroke occurred in recent six months were grouped as symptomatic group. The degree of carotid stenosis was studied with ultrasonography, computed tomography angiography (CTA), or magnetic resonance imagines (MRI).
Carotid endarterectomy (CEA) and atherosclerotic plaque
The study was approved by China-Japan Friendship Hospital Ethical Committee. After pre-surgical examination, informed written consent was obtained from all patients for standard CEA procedure and the collection of specimens for pathological study. The patients were informed the surgical procedures, alternative management, and necessity of pathological study for the carotid plaques. The patients received the conventional CEA; carotid atherosclerotic plaques were removed and collected during surgery. The serial sections of the CEA specimens were taken from the same region of plaques at internal carotid artery around 2-4 mm above the bifurcation of internal carotid artery from common carotid artery.
Pathological study and immunohistochemistry
All plaque specimens were studied pathologically. The surgically removed carotid plaques were fixed in 10% formalin solution for 24 hours, embedded in paraffin, sectioned, and stained with HE and Masson Trichrome staining. In addition, immunohistochemistry was performed for macrophages (CD68), neovascularization (CD105), and inflammatory factor NF-κBp65 (IMG-150A, Imgenex, San Diego, CA, USA), the dilution factor was 1:200 for these three antibodies. Morphology of plaque, size of necrotic lipid core, crystal of cholesterol, degree of calcification, continuity and thickness of fibrous cap were observed.
During the pathological study, the thickness of fibrous cap and area of lipid-rich necrotic core were measured under microscope. The carotid plaques were grouped into stable and unstable plaques based on the thickness of fibrous cap of plaques and area of lipid-rich necrotic core. An unstable plaque was defined as the fibrous cap thickness <65 μm with necrotic lipid core area >40% (18,19). Morphometry was performed with Olympus BX51 semiautomatic image analysis software (DP70) (Nikon Japan). Each slide was scanned under microscopy to determine the interested sites, six separated areas in each slide were selected, and lipid core area and the thickness of fibrous cap in plaque were measured under microscope equipped with Image-Pro Plus 5.1 software. Densitometry analysis was performed for quantification, yielding data in arbitrary units. The results are expressed as average integrated optimal density (IOD) per unit area.
Statistical analysis
SPSS software 13.0 was used for statistical analysis. Differences in degree of stenosis between groups were analyzed using the chi-square test. The image data including density of positive staining area, IOD were expressed as mean with standard error (SE), and were analyzed by student’s t-test. Categorical variables were assessed by Chi-squre test, and Fisher’s exact test. A probability value <0.05 was considered statistically significant.
Results
Basic clinical conditions of patients
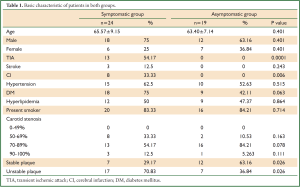
In symptomatic group, all patients had either TIA, stroke or cerebral infarction on CT scan; whereas patients in asymptomatic group had no TIA or stroke, and cerebral infarction on CT scans(P<0.05, Table 1), the incidence of unstable plaque (70.83%, 17/24) in symptomatic patients was higher than that (36.84%, 7/19) in asymptomatic patients (P=0.026). No statistical difference in both groups was found in other demographic and clinical characteristics except TIA and cerebral infarction events (P<0.05, Table 1).
Full table
Pathological studies
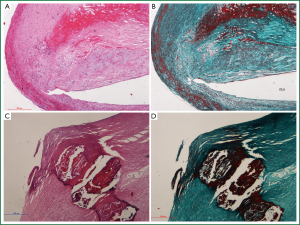
The hemorrhage and necrosis were observed in the carotid plaques (Figure 1). The proportion of hemorrhage and or necrosis was higher in unstable group than stable group (15/24 vs. 5/19, P=0.018). Necrotic lipid core was located in the central part of stable plaque, whereas diffuse necrotic lipid core was close to the intima in unstable plaque. Accumulation of neutrophiles and hemorrhage were found more in unstable plaques than stable plaques (Figure 2).
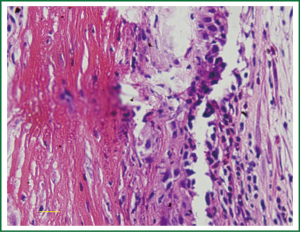
Immunohistochemistry study
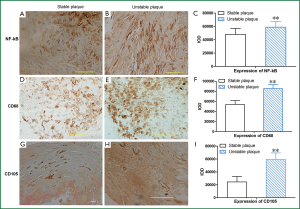
Expression of NF-κB increased in unstable plaques than in stable plaques (P<0.001) (Table 2, Figure 3). The IOD of expressed CD68 in unstable plaques was significantly more than in stable plaques (P<0.001) (Table 2, Figure 3). The staining of CD105 in unstable plaques was significantly higher than in stable plaques (P<0.001) (Table 2, Figure 3).

Full table
Discussion
Our study suggests the presence of more unstable plaques in symptomatic patients with carotid stenosis than in asymptomatic patients. The expression of macrophage and presence of neovascularization are associated with the unstable plaque; the thickness of fibrous cap might predict the stability of plaques. Our results may explain that the unstable plaques cause more non-infective inflammation and subsequently induce the macrophage infiltration and neovascularization.
The development of carotid atherogenic plaque is a chronic non-infective inflammatory process characterized by angiogenesis and inflammatory cell infiltration and hemorrhagic complications (20). It is reported that the expression of NF-κB is related to inflammation of artherosclerotic plaque (21). Our data showed that the NF-κB activation in unstable plaque was higher than in stable plaque, this indicates that more inflammation occurred in unstable plaque than stable plaque.
Macrophages migrate into the plaque area as the progression of plaque. Plaque macrophages represent the majority of leukocytes in the atherosclerotic lesions, and their secretory activity may be related to the fragilization of the fibrous cap and then to the rupture of the plaque (22). Immunohistochemistry showed that plaques from the symptomatic patients had more expression of M1 macrophages (CD68-, CD11c-positive) while plaques from the asymptomatic patients expressed more M2 macrophages (CD163-positive) (23). M1 macrophage content of atherosclerotic plaques is related with incidence of ischemic stroke and increased inflammation or fibrinolysis (22). CD68 (pan macrophage maker) was overexpressed in symptomatic compared with asymptmatic plaques (7). CD68 in fibrocytes are derived from monocytes and contribute to the formation of the fibrous cap (24). While the levels of CD68 in general negatively correlate with plaque cap thickness (25). The increased p53 level in macrophage is associated with the expansion of necrotic cores, plaque rupture and clinical manifestations of carotid plaques.
In consistent with literature reported (6), CD68-positive macrophages in plaque shoulder dominated in unstable plaque of the arterial wall. The number of smooth muscle actin-positive smooth muscle cells decreased in the fibrous cap of atheroma. In present study, the expression of CD105 in unstable plaques was more than those with stable plaques. The neovascularization is increased significantly in diabetic patients (24). It is reported that the atherosclerotic plaque develops from the gradual deposition of lipid underlying of intima; there is a non-infective inflammatory process in the formation of plaque. In general, inflammation related genes such as CD68 are overexpressed in unstable plaques in comparison with stable plaques.
The proportion of unstable plaques in symptomatic patients with carotid stenosis was higher than asymptomatic patients, a high amount of large lipid cores and more calcified plaques were observed in plaque of symptomatic patients. The vulnerable plaque in symptomatic patients may increase the risk of rupture and cause stroke if the patients with vulnerable plaque were not treated.
The limitation of this study is that the number of patients is not large, the normal artery as the control specimens is not available, and the type of macrophage was not identified, and VEGF should be used to evaluate neovascularization in further study.
Overall, our study demonstrates that patients with vulnerable plaques are apt to be symptomatic, and inflammatory mediators including NF-κB, macrophage specific CD68, and neovascularization CD105 are highly expressed in unstable plaques compared with stable plaques. These results are potentially essential from a fundamental standpoint because they may play a crucial role in the destabilization of atherosclerotic lesions in carotid stenosis. From a practical standpoint, these findings provide further support for the necessity of endarterectomy for patients with carotid atherosclerotic stenosis and searching the way to stabilize the unstable carotid plaque via controlling these inflammatory factors.
Acknowledgements
Dr. Ren is the guarantor for this article, and takes responsibility for the integrity of the work as a whole. This paper was supported in part by China International Cooperation Grant (No. 2013DFA31900).
Disclosure: The authors declare no conflict of interest.
References
- Ren S, Liu P, Ma G, et al. Long-term outcomes of synchronous carotid endarterectomy and coronary artery bypass grafting versus solely carotid endarterectomy. Ann Thorac Cardiovasc Surg 2012;18:228-35. [PubMed]
- Van Lammeren GW, Reichmann BL, Moll FL, et al. Atherosclerotic plaque vulnerability as an explanation for the increased risk of stroke in elderly undergoing carotid artery stenting. Stroke 2011;42:2550-5. [PubMed]
- Vavuranakis M, Sigala F, Vrachatis DA, et al. Quantitative analysis of carotid plaque vasa vasorum by CEUS and correlation with histology after endarterectomy. Vasa 2013;42:184-95. [PubMed]
- Kadoglou NP, Sailer N, Moumtzouoglou A, et al. Aggressive lipid-lowering is more effective than moderate lipid-lowering treatment in carotid plaque stabilization. J Vasc Surg 2010;51:114-21. [PubMed]
- Wang J, Razuvaev A, Folkersen L, et al. The expression of IGFs and IGF binding proteins in human carotid atherosclerosis, and the possible role of igf binding protein-1 in the regulation of smooth muscle cell proliferation. Atherosclerosis 2012;220:102-9. [PubMed]
- Gao T, He X, Yu W, et al. Atherosclerotic plaque pathohistology and classification with high-resolution mri. Neurol Res 2011;33:325-30. [PubMed]
- Razuvaev A, Ekstrand J, Folkersen L, et al. Correlations between clinical variables and gene-expression profiles in carotid plaque instability. Eur J Vasc Endovasc Surg 2011;42:722-30. [PubMed]
- Pelisek J, Well G, Reeps C, et al. Neovascularization and angiogenic factors in advanced human carotid artery stenosis. Circ J 2012;76:1274-82. [PubMed]
- Yuan XM, Osman E, Miah S, et al. P53 expression in human carotid atheroma is significantly related to plaque instability and clinical manifestations. Atherosclerosis 2010;210:392-9. [PubMed]
- Chowdhury M, Ghosh J, Slevin M, et al. A comparative study of carotid atherosclerotic plaque microvessel density and angiogenic growth factor expression in symptomatic versus asymptomatic patients. Eur J Vasc Endovasc Surg 2010;39:388-95. [PubMed]
- Pilarczyk K, Sattler KJ, Galili O, et al. Placenta growth factor expression in human atherosclerotic carotid plaques is related to plaque destabilization. Atherosclerosis 2008;196:333-40. [PubMed]
- Shaikh S, Brittenden J, Lahiri R, et al. Macrophage subtypes in symptomatic carotid artery and femoral artery plaques. Eur J Vasc Endovasc Surg 2012;44:491-7. [PubMed]
- Marzullo A, Ciccone MM, Covelli C, et al. Macrophages and mast cells are involved in carotid plaque instability. Rom J Morphol Embryol 2011;52:981-4. [PubMed]
- Martín-Ventura JL, Blanco-Colio LM, Muñoz-García B, et al. NF-kappab activation and Fas ligand overexpression in blood and plaques of patients with carotid atherosclerosis: potential implication in plaque instability. Stroke 2004;35:458-63. [PubMed]
- Jiang X, Dong S, Liao Y, et al. The role of NF-kappa B and I-kappa B in intimal proliferation following balloon catheter-induced injury in the rat carotid artery. J Huazhong Univ Sci Technolog Med Sci 2008;28:33-6. [PubMed]
- Olson FJ, Strömberg S, Hjelmgren O, et al. Increased vascularization of shoulder regions of carotid atherosclerotic plaques from patients with diabetes. J Vas Surg 2011;54:1324-1331.e5.
- Menezes LJ, Kotze CW, Agu O, et al. Investigating vulnerable atheroma using combined (18)F-FDG PET/CT angiography of carotid plaque with immunohistochemical validation. J Nucl Med 2011;52:1698-703. [PubMed]
- Faggioli GL, Pini R, Mauro R, et al. Identification of carotid ‘vulnerable plaque’ by contrast-enhanced ultrasonography: correlation with plaque histology, symptoms and cerebral computed tomography. Eur J Vasc Endovasc Surg 2011;41:238-48. [PubMed]
- Alsheikh-Ali AA, Kitsios GD, Balk EM, et al. The vulnerable atherosclerotic plaque: scope of the literature. Ann Intern Med 2010;153:387-95. [PubMed]
- Slevin M, Turu MM, Rovira N, et al. Identification of a ’snapshot’ of co-expressed angiogenic markers in laser-dissected vessels from unstable carotid plaques with targeted arrays. J Vasc Res 2010;47:323-35. [PubMed]
- Figueroa AL, Subramanian SS, Cury RC, et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging 2012;5:69-77. [PubMed]
- Businaro R, Tagliani A, Buttari B, et al. Cellular and molecular players in the atherosclerotic plaque progression. Ann N Y Acad Sci 2012;1262:134-41. [PubMed]
- Cho KY, Miyoshi H, Kuroda S, et al. The phenotype of infiltrating macrophages influences arteriosclerotic plaque vulnerability in the carotid artery. J stroke Cerebrovasc Dis 2013;22:910-8. [PubMed]
- Medbury HJ, Tarran SL, Guiffre AK, et al. Monocytes contribute to the atherosclerotic cap by transformation into fibrocytes. Int Angiol 2008;27:114-23. [PubMed]
- Medbury HJ, James V, Ngo J, et al. Differing association of macrophage subsets with atherosclerotic plaque stability. Int Angiol 2013;32:74-84. [PubMed]