Effectiveness of high-flow nasal cannula oxygen therapy for acute respiratory failure with hypercapnia
Introduction
High-flow nasal cannula (HFNC) oxygen therapy has been gaining attention as an innovative respiratory support for critically ill patients, especially those with hypoxemic respiratory failure (1-6). It delivers adequately heated and humidified medical gas at flow rates of up to 60 L/min, and is considered to have a number of physiological effects, including reduction of anatomical dead space, positive end-expiratory pressure (PEEP; range, 3–5 cmH2O) effects, maintenance of a constant fraction of inspired oxygen (FiO2), and good humidification (7-9). In a previous multicenter randomized controlled trial (RCT) that was conducted on patients with non-hypercapnic acute hypoxemic respiratory failure, HFNC oxygen therapy did not result in significantly different intubation rates compared with standard oxygen therapy or noninvasive ventilation (NIV), but showed significantly different 90-day mortality (6). Several recent RCTs have revealed that the use of HFNC oxygen therapy reduces the risk of reintubation compared with conventional oxygen therapy or NIV (10,11). Furthermore, a recent meta-analysis has reported that HFNC oxygen therapy modestly reduces intubation rate and intensive care unit (ICU) mortality (12).
In the above-mentioned studies, HFNC oxygen therapy was shown to be beneficial in case of acute respiratory failure; however, these studies excluded patients with hypercapnia (6,12). NIV is universally recognized as the first-line therapy for acute respiratory failure with hypercapnia (13-15). However, NIV via a face mask is not tolerated by every patient due to a variety of side effects or intolerance, such as claustrophobia, eye irritation, pressure sore, and clinical failure (16). So far, there is no established alternative for patients who cannot tolerate standard NIV. Several recent studies suggested that HFNC oxygen therapy is effective in patients with hypercapnia, and demonstrated improvement in ventilatory parameters (17-19). One study revealed a decrease in capillary pCO2 with the use of HFNC, along with a decrease in respiratory rate and minute volume (18). Furthermore, a flow-dependent reduction in the partial pressure of arterial carbon dioxide (PaCO2) with HFNC oxygen therapy was noted in other studies (17,19), suggesting that it may be effective in acute hypercapnic respiratory failure. However, these studies were conducted on patients with a stable status.
Hence, in this study, we investigated the feasibility of the use of HFNC oxygen therapy in patients with acute respiratory failure accompanied by hypercapnia who were admitted to the medical intensive care unit (MICU).
Methods
Study patients
This retrospective study included patients admitted to the MICU of a tertiary teaching university hospital from April 2011 to February 2013. Among patients with acute respiratory failure who were treated with HFNC oxygen therapy, the patients who fulfilled the following criteria were included: (I) a respiratory rate >25 breaths per minute; (II) a ratio of the partial pressure of arterial oxygen (PaO2) to the FiO2 (P/F) ≤300 mmHg with the patient breathing oxygen at a flow rate ≥10 L/min for at least 15 min; (III) PaCO2 >45 mmHg and available arterial blood gas analysis (ABGA) results 1 and/or 24 h after HFNC application during routine clinical practice in the ICU. Chronic hypercapnia was defined as PaCO2 >45.0 mmHg recorded twice with an interval of at least 6 weeks. The main exclusion criteria were severe neutropenia, hemodynamic instability, use of vasopressors, a Glasgow Coma Scale score of 12 points or less (on a scale of 3 to 15, with lower scores indicating reduced levels of consciousness), contraindication to NIV, urgent need for endotracheal intubation, and a do-not-intubate order. The study was approved by the Institutional Review Board and Ethics Committee (IRB No. B-1708-414-110) and was conducted in compliance with the Declaration of Helsinki. The requirement for informed consent to approve patient record data analysis was waived by the Ethics committee.
Study design and outcomes
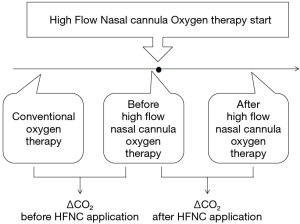
HFNC (OptiflowTM, Fisher & Paykel Healthcare) was first implemented in the MICU in April of 2011, and Figure 1 shows the flow of patients applied with HFNC. Initially, the patients received conventional treatment including oxygen therapy to treat hypoxemia. Conventional oxygen therapy was defined as oxygen therapy via nasal prongs, facial mask, or NIV, and the rate was adjusted to maintain an oxygen saturation level of 90% or more, as measured by pulse oximetry (SpO2), until the patient recovered or HFNC was applied. HFNC was applied when the patients showed no clinical improvement with conventional treatment or in case of intolerance to NIV. Clinical failure to the conventional oxygen therapy was defined and judged within 30 minutes if the patients show any aggravation of indexes on respiration including respiratory rate per minute, use of accessory muscle, oxygen saturation level or PaO2, PaCO2, pH on ABGA. HFNC oxygen therapy was applied continuously through large-bore binasal prongs, with a gas flow rate of 50 L/min and an FiO2 of 1.0 at initiation. The fraction of oxygen in the gas flowing in the system was subsequently adjusted to maintain blood oxygen saturation levels (SpO2) of 90% or more. If patients were tolerable, HFNC was applied for at least 1 day, after which it could be stopped and conventional oxygen therapy started, depending on the patient’s condition. Respiratory variables were recorded and arterial blood gas analyses conducted at the time of admission to the ICU, and before, and at 1 and 24 h (if available) after initiation of HFNC oxygen therapy related with routine clinical practice in the ICU. The primary outcome was the change in PaCO2 after HFNC application in 1 and 24 h, compared to that achieved with conventional oxygen therapy. The secondary outcomes included pH changes in ABGA, vital signs, and oxygenation variables. In addition, we assessed the patients’ demographic and clinical characteristics, and the information on cause of respiratory failure and in-hospital death via a medical chart review.
Statistical analysis
Descriptive data are expressed as mean ± standard deviation, unless otherwise specified. Student’s t-test or paired t test was used to compare continuous variables, and chi-square or Fisher’s exact tests were used to compare categorical variables. Unless otherwise noted, all tests were two-sided and performed at a significance level of 0.05. Analyses were performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
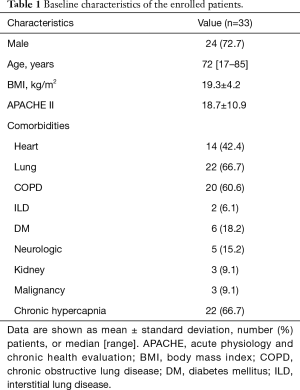
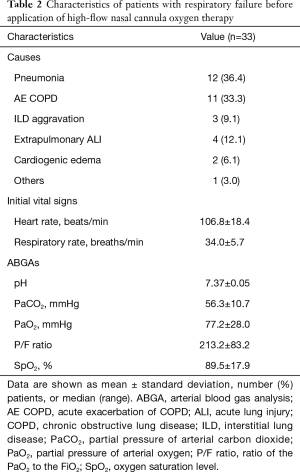
Among 194 patients who admitted to the MICU due to acute respiratory failure during the study period, 40 patients were diagnosed with hypercapnic respiratory failure. Seven patients were excluded from the study because the absence of ABGA results 1 or 24 h after HFNC application. As a result, 33 patients were finally included in the study. Their characteristics are summarized in Table 1. The mean patient age was 72.0 years (range, 17–85 years), and 24 (72.7%) patients were male. More than half of the patients had chronic lung disease (66.7%), and chronic obstructive pulmonary disease (COPD; 60.7%) was the most common condition. Twenty-two (66.7%) patients had chronic hypercapnia. Table 2 shows the additional characteristics of the patients, including the causes of respiratory failure. Most of them were admitted to the ICU due to respiratory failure caused by pneumonia (36.4%) or acute exacerbation of COPD (33.3%). The initial respiratory rate was 34.0±5.7 breaths/min and the initial ABGA revealed that the mean PaCO2 was 56.3±10.7 mmHg and P/F ratio was 213.2±83.2.
Full table
Full table
Clinical assessment and outcomes
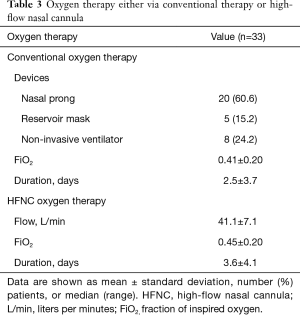
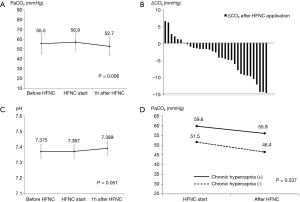
The conventional oxygen therapy administered just before applying HFNC is summarized in Table 3. All patients received conventional oxygen therapy to treat hypoxemia at ICU admission. But, one patient was changed to room air from oxygen therapy via nasal prongs just before HFNC application due to aggravation of hypercapnia. Most of the patients received oxygen therapy via nasal prongs (60.6%) and NIV (24.2%). Baseline PaCO2 just before the initiation of HFNC oxygen therapy was 55.0±12.2 mmHg; it increased by about 1.0±7.7 mmHg with conventional oxygen therapy before HFNC was applied. Three patients were not tolerable to HFNC oxygen therapy and the median duration of HFNC application was 5.2 (2.5–8.7) h. With the application of HFNC, PaCO2 decreased by 4.2±5.5 and 3.7±10.8 mmHg in 1 and 24 h, respectively. Compared to conventional oxygen therapy, HFNC oxygen therapy led to a significant improvement in hypercapnia (P=0.006 and 0.062 after 1 and 24 h, respectively). Figure 2 shows the changes in PaCO2 and pH with HFNC oxygen therapy. Most of the patients showed significant decreases in PaCO2 after initiation of HFNC oxygen therapy (P=0.006). pH measured by ABGA improved with HFNC therapy, but the difference was not significant (P=0.051). When analyzing patients according to presence or absence of chronic hypercapnia, HFNC oxygen therapy was effective irrespective of whether chronic hypercapnia was present or not; the two groups showed similar trends (P=0.537). Even though clear trends of stabilization of heart rate and respiratory rate, and improvement of oxygenation were noticed, the changes were not statistically significant. Table 4 shows detailed information on PaCO2 changes after HFNC application according to the etiology of respiratory failure. All patients showed clear improvement in hypercapnia after HFNC application, except the patients with extrapulmonary ALI and sleep apnea. Furthermore, we analyzed our data to determine whether the beneficial effect of HFNC in hypercapnia was different for each conventional oxygen modalities. The PaCO2 differences after 1 h HFNC application were −4.9±5.5 for nasal prong, −3.4±4.5 for facial mask, and −3.1±6.4 mmHg for NIV (P=0.697). The PaCO2 differences after 24 h HFNC application) was −5.8±2.6 for nasal prong, −2.3±5.1 for facial mask, and 0.6±2.6 mmHg for NIV (P=0.360). No significant difference was noted between them, but HFNC oxygen therapy seemed to be more effective in the patients with nasal prong or facial mask than those with NIV. Four patients required invasive mechanical ventilation after HFNC oxygen therapy. However, no one showed aggravated hypercapnia. Five patients (15.2%) died during the study period; respiratory failure was the most common cause of death (80%).
Full table

Full table
Discussion
In this study, we investigated the effectiveness of HFNC oxygen therapy in patients with acute respiratory failure accompanied by hypercapnia who were admitted to the MICU. This study revealed that HFNC oxygen therapy was beneficial even in the patients with acute hypercapnic respiratory failure. The vital signs were stabilized and oxygenation variables like PaO2 or FiO2 was improved after HFNC oxygen therapy. More importantly, HFNC was useful in the improvement of hypercapnia. HFNC oxygen therapy led to a significant improvement in hypercapnia after 1 h and after 24 h even though statistical significance was shown only in 1 h of HFNC initiation (P=0.006), not in 24 h (P=0.062). In this study, HFNC was applied continuously with a gas flow rate of 50 L/min at initiation and was maintained for an hour. However, the mean flow rate changed to 40.0±8.5 L/min after 24 h and only 10 patients were applied with a gas flow rate more than 50 L/min. Previous study reported that a linear positive correlation between tracer-gas clearance in the model and the flow rate of HFNC, approximately 1.8 mL/s increase in clearance for every 1.0 L/min increase in flow (20). The readjusted and decreased flow rate of HFNC in 24 h could be a plausible explanation for it. PaCO2 and pH was significantly improved with HFNC oxygen therapy irrespective of whether chronic hypercapnia was present or not. HFNC oxygen therapy was applied in about a quarter of the patients (24.2%) included in this study, due to inability to tolerate NIV, similar to real clinical practice. The patients reported that they disliked their experience with the mask. They noted that it made them claustrophobic with feelings of suffocation and loss of control, which made it difficult for them to relax. The vital signs and oxygenation parameters were not aggravated and they wanted to discontinue NIV because of inconvenience. Therefore, we applied HFNC oxygen therapy instead of endotracheal intubation. These patients showed a significant improvement in hypercapnia, and other variables, including vital signs and oxygenation parameters, also showed improvements after application of HFNC, but without statistical significance. Though these results are those of a pilot study, they provide supporting evidence to conduct further interventional studies to investigate the use of HFNC oxygen therapy for acute hypercapnic respiratory failure.
Several hypotheses have been proposed to explain the improvements in hypercapnia observed after initiation of HFNC oxygen therapy. Fricke et al. showed that HFNC can improve hypercapnia in some patients through clearance of anatomical dead space, which improves alveolar ventilation, thus leading to reduction of PaCO2 (17). Bräunlich et al. showed a flow-dependent reduction in PaCO2; they also believed that it was achieved by a washout of the respiratory tract and a functional dead space (19). Further follow up studies are needed to confirm these findings and apply them to clinical practice.
Generally, patients with COPD who receive supplemental oxygen are susceptible to CO2 narcosis because of ventilation/perfusion matching, the Haldane effect, and respiratory homeostasis (21,22). In our study, more than half of the patients had COPD and their PaCO2 indeed increased by 1 mmHg after initiation of conventional oxygen therapy. However, they showed dramatic decreases in PaCO2 after initiation of HFNC oxygen therapy, which is in line with the results of previous studies that were conducted in stable COPD patients (18,19). Follow-up studies for the comparison of HFNC and NIV in acute exacerbation of COPD would be interesting.
Our study has a few limitations. First, this was a single-center retrospective study including a small number of patients. Second, it was not a comparative study; we did not compare our results with those in a cohort that did not receive HFNC oxygen therapy. Lastly, we applied HFNC for very short durations, and our study period was also short (approximately 2 years). However, these limitations are due to the fact that HFNC oxygen therapy is not a standard therapy as of now, and this study was conducted as a preliminary study to check the feasibility of the use of HFNC oxygen therapy for acute hypercapnic respiratory failure before conducting further studies, including RCTs.
Conclusions
HFNC oxygen therapy with sufficient FiO2 to maintain a normal PaO2 resulted in a significant reduction in PaCO2 in patients with acute respiratory failure accompanied by hypercapnia. Further large-scale RCTs are required to evaluate the efficacy of this therapy in clinical settings.
Acknowledgments
The authors acknowledge the patients with acute respiratory failure who allow us to conduct clinical research studies in an effort to improve the lives of those patients. We also acknowledge the insightful contributions of Se Joong Kim MD; Jisoo Park MD; Yeon Joo Lee, MD; Jong Sun Park, MD; Ho Il Yoon, MD; Jae Ho Lee, MD; Choon-Taek Lee, MD; and Young-Jae Cho MD.
Funding: This work was supported by Seoul National University Bundang Hospital general research fund No. 02-2011-055.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board and Ethics Committee (IRB No. B-1708-414-110) and was conducted in compliance with the Declaration of Helsinki. The requirement for informed consent to approve patient record data analysis was waived by the Ethics committee.
References
- Cuquemelle E, Pham T, Papon JF, et al. Heated and humidified high-flow oxygen therapy reduces discomfort during hypoxemic respiratory failure. Respir Care 2012;57:1571-7. [Crossref] [PubMed]
- Frat JP, Brugiere B, Ragot S, et al. Sequential application of oxygen therapy via high-flow nasal cannula and noninvasive ventilation in acute respiratory failure: an observational pilot study. Respir Care 2015;60:170-8. [Crossref] [PubMed]
- Roca O, Riera J, Torres F, et al. High-flow oxygen therapy in acute respiratory failure. Respir Care 2010;55:408-13. [PubMed]
- Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med 2011;37:1780-6. [Crossref] [PubMed]
- Sztrymf B, Messika J, Mayot T, et al. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: a prospective observational study. J Crit Care 2012;27:324 e9-13.
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185-96. [Crossref] [PubMed]
- Nishimura M. High-flow nasal cannula oxygen therapy in adults. J Intensive Care 2015;3:15. [Crossref] [PubMed]
- Parke RL, McGuinness SP, Eccleston ML. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care 2011;56:265-70. [Crossref] [PubMed]
- Dewan NA, Bell CW. Effect of low flow and high flow oxygen delivery on exercise tolerance and sensation of dyspnea. A study comparing the transtracheal catheter and nasal prongs. Chest 1994;105:1061-5. [Crossref] [PubMed]
- Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA 2016;315:1354-61. [Crossref] [PubMed]
- Hernández G, Vaquero C, Colinas L, et al. Effect of Postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA 2016;316:1565-74. [Crossref] [PubMed]
- Liesching TN, Lei Y. Efficacy of high-flow nasal cannula therapy in intensive care units. J Intensive Care Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Lightowler JV, Wedzicha JA, Elliott MW, et al. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 2003;326:185. [Crossref] [PubMed]
- Ambrosino N, Vagheggini G. Non-invasive ventilation in exacerbations of COPD. Int J Chron Obstruct Pulmon Dis 2007;2:471-6. [PubMed]
- Mitrofan EC, Steciu A, Rusu G. Non invasive ventilation in acute hypercapnic exacerbations from COPD. Pneumologia 2009;58:13-8. [PubMed]
- Gay PC. Complications of noninvasive ventilation in acute care. Respir Care 2009;54:246-57; discussion 257-8. [PubMed]
- Fricke K, Tatkov S, Domanski U, et al. Nasal high flow reduces hypercapnia by clearance of anatomical dead space in a COPD patient. Respir Med Case Rep 2016;19:115-7. [Crossref] [PubMed]
- Braunlich J, Seyfarth HJ, Wirtz H. Nasal High-flow versus non-invasive ventilation in stable hypercapnic COPD: a preliminary report. Multidiscip Respir Med 2015;10:27. [Crossref] [PubMed]
- Bräunlich J, Köhler M, Wirtz H. Nasal highflow improves ventilation in patients with COPD. Int J Chron Obstruct Pulmon Dis 2016;11:1077-85. [Crossref] [PubMed]
- Möller W, Celik G, Feng S, et al. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol (1985) 2015;118:1525-32. [PubMed]
- Kim V, Benditt JO, Wise RA, et al. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:513-8. [Crossref] [PubMed]
- Agusti AG, Carrera M, Barbé F, et al. Oxygen therapy during exacerbations of chronic obstructive pulmonary disease. Eur Respir J 1999;14:934-9. [Crossref] [PubMed]