Radical treatment for left upper-lobe cancer via complete VATS
Introduction
Video-assisted thoracic surgery (VATS) was initially introduced in early 1990s. In 1992, Lewis completed the first VATS lobectomy (1). After twenty years of development, VATS has been regarded as one of the greatest breakthroughs in the late 20th century. There are two leaps during its development: from simultaneous stapling to anatomic lobectomy; and from small auxillary incision to complete VATS (2). Upon the joint efforts of global thoracic surgeons, VATS was, for the first time, cited in the 2006 US NCCN guidelines on lung cancer—“VATS is a feasible option for resectable lung cancer, particularly for patients who can not tolerate the standard open surgery due to physical status and other reasons”. It is feasible for phase I lung cancer. In 2012, VATS, as the widely recognized standard treatment for non-small cell lung cancer (NSCLC), can be applied in carefully selected patients who have no anatomic or surgical contraindications. Thus, the indications of VATS are extended for tumors other than phase I lung cancer.
In China, VATS technique was introduced in 1992, and then witnessed the early stage [1992-1994], the growth stage [1995-1999], and the steady stage [2000-2005]. Currently, VATS has been widely applied for the treatment of lung cancer and other common thoracic diseases, indicating that the development of VATS has entered its mature stage. Compared with the conventional open surgery, the radical treatment of lung cancer via VATS shows no significant differences in terms of operative time, intraoperative blood loss, and number of the dissected lymph nodes during the surgery. The VATS has many advantages: small surgical trauma; protection of lung function; reduction in post-operative pain; quick recovery; shorter hospital stay and lower healthcare cost; and is particularly feasible for high-risk patients (3,4).
The common procedures of VATS lobectomy include anatomic lobectomy, single-direction lobectomy, simultaneous stapling, and retrograde lobectomy. During the anatomic lobectomy, the pulmonary artery or vein is divided and resected firstly, followed by the bronchus. In the single-direction lobectomy, anatomy is performed from the most shallow structures at the hilum of lung, then expose and divide the other structures in the same direction; finally, the lung fissure is handled (5). Simultaneous stapling does not need to expose the structures at the hilum of lung such as pulmonary artery, pulmonary vein and bronchus; it uses a linear cutter to staple and cut the structures, which can be associated with high risk and many complications (1). During the retrograde lobectomy, the operator needs to transect the bronchus, and thus handles the pulmonary artery or pulmonary vein. This procedure is particularly useful for patients with large tumors or swollen lymph nodes, in whom the pulmonary artery can not be easily exposed; once it is accidentally damaged or bleeds, total pneumonectomy may become unavoidable, or the surgery can not be smoothly completed. Therefore, the bronchus must be transected firstly, so as to alleviate the oppression of tumor or lymph node on the pulmonary artery. Once the hilum of lung loses the fixation of the hard bronchus and is only adhered to the connective tissue, the branches of pulmonary artery can be easily handled. By doing so, this procedure is helpful to lower the risks and increase the success rate of the surgery.
This article describes a patient with left upper lung cancer who received retrograde lobectomy in the department of thoracic surgery, the First Affiliated Hospital of Guangzhou Medical College, due to the presence of swollen lymph nodes at the hilum of lung (Figures 1,2).
Clinical data
A 60-year-old female patient was admitted due to “shadow in the left upper lung” during health screening. The patient had a history of type 2 diabetes for ten years, and at the time of diagnosis her blood sugar was well controlled by using oral hypoglycemic agents.
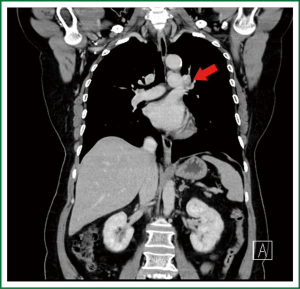
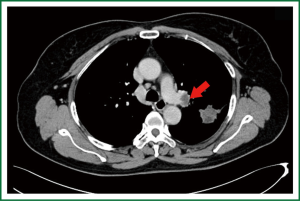
Chest CT indicated that there was a patchy dense shadow sized 4.0 cm × 2.8 cm at the apico-posterior segment of left upper lobe. The mass had relatively clear margin and uneven density, with small vacuoles and flaky low-density shadow inside. Contrast agent-enhanced CT showed edge enhancement, with no obvious enhancement in the central low-density area. Burry edge was found, and the adjacent pleura was slightly thickened. A lymph node sized 2.0 cm × 1.5 cm was seen at the left pulmonary hilum, showing ring enhancement. Brain contrast-enhanced MR/whole body bone scan/abdominal contrast-enhanced CT showed no signs of tumor metastasis.
The preoperative diagnosis was left lung T2aN1M0, stage II.
Lung function: FEV1 1.99 L, 99% of predicted.
Cardiac function: cardiac systolic function, normal; EF, 72%.
Preoperative assessment
For patients with the initial diagnosis of lung cancer, brain contrast-enhanced MR/whole body bone scan/abdominal contrast-enhanced CT should be performed to rule out the distant metastasis of tumors before the surgery. Also, the cardiopulmonary functions should be evaluated. Furthermore, assessment of pulmonary ventilation function, cardiac function, and coronary CT are also necessary. Patients with good pulmonary ventilation function (FEV1 greater than 1.5 L, FEV1/FVC greater than 50%, and the postoperative expected FEV1 greater than 0.8 L) are relatively safe to receive lobectomy.
In our current patient, the swollen lymph node at the left pulmonary hilum compressed the left pulmonary artery. The tumor was located between the artery branch at the apical segment of left upper lobe and the posterior segment of the arterial branch, and the surgery could be challenging. There was still a risk for left pneumonectomy. Before the surgery, we informed the patient and her family about the disease conditions and all the possibilities during the surgery.
Video description
Anesthesia and position
Conventional double lumen endobronchial tube was applied for anesthesia, with the patient lying on her right side.
Surgical incisions (Figure 3)
During the complete VATS, a 3.5-cm main operation port was made in the 4th intercostal space at the left middle axillary line; a 1.0-cm observation port was made in the 6th intercostal space at the middle axillary line; and a 1.0-cm auxiliary port was made in the 6th intercostal space at the left middle axillary line. All the ports were interchanged during the surgery based on the real conditions.
Surgical steps (Video 1)
- After the pulmonary fissure was divided, wedge resection of the left lung was performed. Biopsy of the intraoperative frozen sections showed that the mass was an invasive adenocarcinoma, and left upper lobectomy was considered.
- After the interlobular artery was isolated from the oblique fissure, the lingual artery segment was explored firstly, and then ligated and transected.
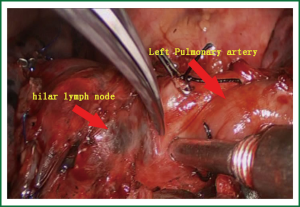
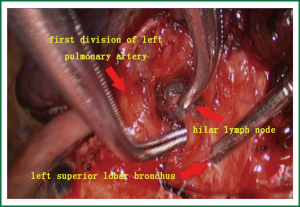
- The relationship between the pulmonary trunk and hilar lymph nodes was explored, and then they were sharply divided using scissors (Figure 4).
- Intraoperative exploration showed that there was a close relationship between the pulmonary trunk and hilar lymph nodes. The operation began from the hilum of lung. First, the lingual vein segment; second, the upper pulmonary vein was ligated and transected, which paved the way for the exploration of the left upper lung bronchus and the first branch of the left upper pulmonary artery.
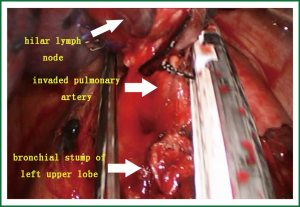
- The first branch of the left upper pulmonary artery can not be directly divided due to hilar lymph node involvement (Figure 5). Therefore, the bronchus was cut open, and retrograde left upper lobe resection was considered. After the bronchus was cut open, the lymphoid tissue between the bronchus and the artery was divided.
- Two branches of the left upper pulmonary artery were divided using a stapler (Figure 6). The specimens were then removed.
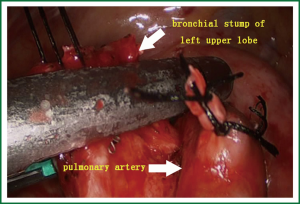
- The stump of the left upper lung bronchus and its surrounding lymph node tissues were divided using scissors. The stump at the opening of the left upper lung bronchus was handled with a stapler (Figure 7).
- Dissection of lymph node stations 5, 7, and 10.
- After adequate hemostasis, two chest tubes (upper and lower) were placed.
Postoperative treatment
After the surgery, the patient was routinely treated with antibiotics, expectorants, and bronchus relaxation agents. The patient was encouraged to expectorate. Three days after the surgery, the chest tubes were removed. The patient was discharged on the 8th postoperative day.
Pathological diagnosis
A sallow mass sized 4 cm × 3 cm × 3 cm in the left upper lung; pleura was not involved; the diagnosis was invasive adenocarcinoma in the left upper lung. After the mass was removed, no residual tumor was found. No cancer was detected at the stumps of bronchus or vessels. Tumor metastasis was seen in three peribronchial lymph nodes (3/3), 3 station 5 lymph nodes (3/4), 0 station 7 lymph node (0/3), 0 station 10 lymph nodes (0/2), and 1 station 11 lymph node (1/1).
Postoperative diagnosis: left lung invasive adenocarcinoma; T2aN2M0; stage III.
Discussion
For patients with swollen hilar lymph nodes, the anterograde anatomy along the conventional sequence of pulmonary artery, pulmonary vein, and bronchus can often be difficult. Furthermore, since the pulmonary vessels are often fragile, forced separation of these structures can result in the rupture and bleeding of these vessels. The retrograde lobectomy can make up for the traditional anterograde lobectomy in terms of safety and surgical resection rate. During this procedure, since the bronchus is located behind the hilum of lung, it can be easily toughed and dissected; once the bronchus is divided, the pulmonary hilum can be adequately released, to expand the operation field for the lung vessels and facilitate the control of vessels within the pericardium. By doing so, it can meet the treatment principles of anatomic lobectomy and avoid pneumonectomy. In addition, complete VATS will not be an obstacle for experienced surgeons. Nevertheless, retrograde resection still has some risks, which should be carefully assessed before surgery. Pulmonary artery, pulmonary vein, and pericardium must be carefully explored during the surgery, and the possibility of sleeve resection or pneumonectomy should be well prepared.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lewis RJ, Sisler GE, Caccavale RJ. Imaged thoracic lobectomy: should it be done? Ann Thorac Surg 1992;54:80-3. [PubMed]
- Congregado M, Merchan RJ, Gallardo G, et al. Video-assisted thoracic surgery (VATS) lobectomy: 13 years’ experience. Surg Endosc 2008;22:1852-7. [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [PubMed]
- Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg 2008;85:S719-28. [PubMed]
- Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. [PubMed]