Granulomatous reaction of primary mediastinal seminoma leading to diagnostic delay: a case report
Introduction
Primary mediastinal seminoma (PMS) is rare among extragonadal germ cell tumors (GCTs). PMS is usually located in the anterior mediastinum and affects young males (1). Among the GCTs arising in the anterior mediastinum, seminoma is the second most common tumor type after mature teratoma, accounting for approximately 37% of all mediastinal GCTs and 25% of extragonadal GCTs (2,3). The diagnosis must be considered after exclusion of primary testicular tumor and can be made with percutaneous needle biopsy alone, unless the tumor is masked by secondary changes in the form of a granulomatous reaction, as was seen in our case. We herein report an interesting case of anterior mediastinal seminoma associated with an abundant granulomatous reaction, which led to a delay in diagnosis for a young male patient.
Case presentation
A 26-year-old man underwent a routine check-up chest X-ray for a pre-recruitment medical examination, which revealed a mediastinal contour abnormality in the region of the right hilum (Figure 1A). He was referred to our hospital without symptoms such as cough, dyspnea, fever, night sweating and weight loss. He was a never-smoker and didn’t have a history of any testicular neoplasm. General physical and systemic examinations were within normal limits. On admission, the vital parameters were as follows: body temperature 36.4 °C, pulse rate 78/min, and blood pressure 116/72 mmHg. There was no lymphadenopathy, no pallor, and no raised jugular venous pressure. Baseline hematological and biochemical parameters were normal. His serum beta-human chorionic gonadotropin (b-hCG) was 1.48 mIU/mL (normal <5.00 mIU/mL) and serum alpha-fetoprotein (AFP) was 1.00 ng/mL (normal <10.00 ng/mL). Sputum Acid-Fast Bacilli (AFB) and serum tuberculosis (TB)-polymerase chain reaction (PCR) were all negative. The ultrasound of the scrotum was unremarkable. Computed tomography (CT) revealed a heterogeneously enhancing lobulated well-defined mass in the anterior mediastinum closely abutting the aorta and right lung. The mass measured a maximum of 3 cm in the longest dimension and showed a small area of a low-density lesion (Figure 1B). There were no focal lesions noted in either lung. A transthoracic CT-guided biopsy was done. A hematoxylin and eosin (H and E) section of the trucut biopsy showed epithelioid cell granulomas in a background of mature lymphocytes (Figure 1C). A special stain Ziehl-Neelsen was done on the aspiration, and biopsy slides were both negative for AFB. No neoplastic cell population was noted on the small biopsy. Based on histomorphological and clinical findings, a differential of TB or sarcoidosis was considered. His serum lactate dehydrogenase (LDH) was 550 U/L (normal range is 200–400 IU/L), and his serum calcium and serum angiotensin-converting enzyme levels were within normal limits.
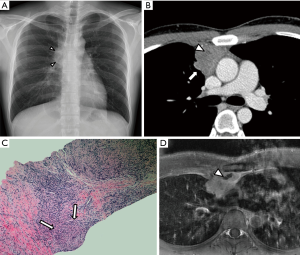
The patient was started on anti-TB treatment. However, on follow-up CT and magnetic resonance imaging (MRI) at 2 months, the anterior mediastinal mass slightly increased in size and a small part of low density or signal intensity appeared on a contrast-enhancement study (Figure 1D). Laterally, the mass was surrounded by the anterior segment of the right upper lobe of the lung, and anteriorly, it was abutting the anterior chest wall. A few subsegmental patchy areas of consolidation and adjacent ground glass opacities were also noted in the subpleural location of the anterior segments of the right upper lobe. Because there was a persistence of mass in the mediastinum with no regression, he was reevaluated clinically for alternate possibilities as well.
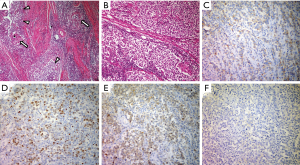
We performed a total resection of the tumor through a median sternotomy. The tumor was fully covered with a fibrous capsule that had a partly-dense adhesion to the left mediastinal pleura. The right mediastinal pleura were resected with the tumor. The pathological diagnosis was seminoma with granulomatous inflammation. Higher magnification showed large polygonal cells with a clear cytoplasm, round or oval nuclei, and prominent nucleoli. The immunohistochemical stain for c-kit, oct3/4, d2-40, and placental-like alkaline phosphatase stain was positive, and a b-hCG stain was negative. So, these cells were diagnosed as seminoma cells (Figure 2).
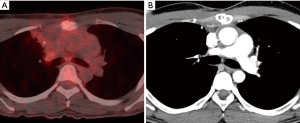
Our patient was classified as an intermediate risk group due to adjacent pulmonary invasion, so he received four courses of VIP (100 mg/m2 etoposide on days 1–5, 100 mg/m2 ifosfamide on days 1–5, and 30 kU cisplatin on days 1, 8, and 15, repeated 21 days) as adjuvant chemotherapy. Follow-up PET (positron emission tomography)/CT and CT scans revealed no recurrence or metastasis for 8 months (Figure 3).
Discussion
PMS is the most common malignant mediastinal GCT, accounting for 25–40% of such tumors (4). It may be associated with a low-level elevation of b-hCG; on the other hand, greater elevation of b-hCG or AFP is diagnostic of a non-seminomatous GCT component (4,5). Elevation of serum LDH is seen in patients with advanced seminoma (4). A common CT finding of PMS is a coarsely-lobulated anterior mediastinal mass, typically with homogeneous density and diffuse contrast-enhancement (4). However, radiologically, it is always challenging to accurately diagnose an anterior mediastinal mass in a young adult. GCT, lymphoma, and thymoma are common disease entities, but the radiographic differences among those diseases are uncertain. Therefore, the tissue diagnosis for accurate diagnosis is essential.
We herein describe a patient with mediastinal seminoma that was initially misdiagnosed as mediastinal TB through a small needle biopsy. On initial CT, considering the patient’s age and tumor location, we suspected a GCT, lymphoma or thymoma. However, all tumor markers, including b-hCG, and AFP were in the normal range, and a small needle biopsy revealed chronic granulomatous inflammation with caseous necrosis. Our findings therefore suggested mediastinal TB. Initial CT showed a small low-density area in the mediastinal mass, and follow-up MRI showed distinct low signal intensity area in Gadolinium enhanced fat-suppressed T1-weighted image. We believe that the lesion pathologically correlated with the granulomatous inflammation. To the best of our knowledge, this is the first report on this pathologic and radiologic correlation.
There are a few case reports about mediastinal seminoma accompanied by secondary changes that may conceal the actual diagnosis, as was seen in our case. These changes include reactive follicular hyperplasia, large cysts, epithelioid granulomas, and fibrosis (6). Our case resulted in a diagnostic dilemma following microscopic examination, as there were no neoplastic cells that were masked by inflammatory cells, along with a florid granulomatous reaction. Only a few mediastinal seminomas with chronic granulomatous inflammation have been reported previously (7-9). There was a case in which only a mediastinal seminoma and chronic granulomatous inflammation were pathologically accompanied, and they were also mistaken from a small biopsy to be TB, and the diagnosis was delayed (8).
The mechanisms responsible for a granulomatous reaction are still unclear. On a histological level, PMS can be associated with a range of secondary changes that may be so prominent as to conceal the underlying neoplasm. Mediastinal seminomas morphologically closely resemble their gonadal counterparts but can also be associated with striking secondary changes. These include the presence of remnant thymic tissues, cystic degeneration, epithelioid granulomatous reaction, fibrotic change, and syncytiotrophoblast-like cells (2,10). From this perspective, the significance of these secondary changes lies in the fact that they may be so extensive as to obscure the seminoma component, and the tumor may be mistaken for a reactive condition or an unrelated neoplasm. A small biopsy may not be representative of the true lesion and depict only peripheral secondary changes, as was seen in our case. If the radiologic findings and pathologic results of a small needle biopsy are not concordant, we suggest that a second needle biopsy or a surgical biopsy should be considered.
We herein report a mediastinal seminoma that was initially misdiagnosed as mediastinal TB from a small needle biopsy. Mediastinal seminoma can manifest with secondary changes, such as necrotic changes represented by granulomatous inflammation. Therefore, awareness of the secondary changes that can mimic a primary lesion can help with an early correction of this misdiagnosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Busch J, Seidel C, Zengerling F. Male extragonadal germ cell tumors of the adult Oncol Res Treat 2016;39:140-4. [Crossref] [PubMed]
- Moran CA, Suster S, Przygodzki RM, et al. Primary germ cell tumors of the mediastinum: II. Mediastinal seminomas--a clinicopathologic and immunohistochemical study of 120 cases. Cancer 1997;80:691-8. [Crossref] [PubMed]
- Dehner LP. Germ cell tumors of the mediastinum. Semin Diagn Pathol 1990;7:266-84. [PubMed]
- Shinagare AB, Jagannathan JP, Ramaiya NH, et al. Adult extragonadal germ cell tumors AJR Am J Roentgenol 2010;195:W274-80. [Crossref] [PubMed]
- Bokemeyer C, Nichols CR, Droz JP, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol 2002;20:1864-73. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Mediastinal seminoma with florid follicular lymphoid hyperplasia: a clinicopathological and immunohistochemical study of six cases. Virchows Arch 2015;466:209-15. [Crossref] [PubMed]
- Ishii H, Igata F, Nabeshima K, et al. Mediastinal Seminoma with an Elevated Level of Serum Angiotensin-converting Enzyme. Intern Med 2015;54:1909-12. [Crossref] [PubMed]
- Gupta D, Rath A, Rathi KR, et al. Primary thymic mediastinal seminoma with florid granulomatous reaction. Indian J Pathol Microbiol 2016;59:351-4. [Crossref] [PubMed]
- Tjan-Heijnen VC, Vlasveld LT, Pernet FP, et al. Coincidence of seminoma and sarcoidosis: a myth or fact? Ann Oncol 1998;9:321-5. [Crossref] [PubMed]
- Moran CA, Suster S. Mediastinal seminomas with prominent cystic changes. A clinicopathologic study of 10 cases. Am J Surg Pathol 1995;19:1047-53. [Crossref] [PubMed]


