Peeling back the onion: addressing nuances of CT screening for lung cancer
At first glance, screening for lung cancer is straightforward. You can find lung cancer at an early stage with a CT scan, so if you do a lot of CT scans you will save a lot of lives. However, the devil is in the details. One of the problems is that a chest CT detects nodules in many patients; deciding when a nodule is just background noise or warrants closer attention is an important component of the screening process. Nuances of how to do this are still being developed.
Study design
A recent paper by Henschke et al. (1) published in European Radiology is worth attention as it provides data that defines important nuances related to screening for lung cancer. This study examines data from 65,372 baseline and 74,482 annual repeat screenings in the International Early Lung Cancer Action Program (I-ELCAP) experience from 1992–2014. This is a prospective systematic database of a multi-institutional experience in CT screening for lung cancer. The study focused on differences in the nature of cancers first noticed in a baseline vs an annual repeat CT scan.
The cancer screening setting is inherently different than the usual care setting in which cancers are found (i.e., due to symptoms). Screening aims to detect (i.e., diagnose) a cancer well before it would lead to symptoms; this difference in the time of diagnosis is known as the lead time. Even if there is no effective treatment and people are destined to die at the same time regardless of when their cancer was detected, the time from diagnosis to death will be longer for screen-detected cancers compared with symptom detected cancers by the duration of this “lead time”.
Another effect of screening is related to the fact that cancers exhibit a spectrum of aggressiveness, from highly aggressive tumors to some that are very indolent. A baseline screening test is more likely to detect very indolent tumors. Because indolent tumors are present for long periods of time, they are more likely to be detected by a baseline screening test, whereas a rapidly growing tumor transits quickly from being potentially detectable by screening to becoming symptomatic. Thus, the act of screening increases the proportion of indolent tumors among all cancers detected. This is sometimes referred to with the rather obscure term “length-time” bias (including in the study by Henschke et al.). I prefer to call this an altered spectrum of aggressiveness, as I think it more intuitively describes the effect.
The study by Henschke et al. seeks to estimate how much screening causes an altered spectrum of aggressiveness (length time bias) and how much lead time (i.e., earlier diagnosis) can be attributed to the screening intervention. The approach used is to compare lung cancers detected by the baseline screening CT and those detected by an annual repeat screening CT. Comparison of the proportion of different types of lung cancer seen in the baseline and the annual repeat screening rounds provides an estimate of the altered spectrum of aggressiveness (slow growing tumors will be much more likely to be incidentally detected during the baseline round, and relatively unlikely to show up as newly discovered cancers on an annual repeat scan). For each particular cell type the ratio between the baseline and annual repeat rounds of the percentage of all detected cancers provides an estimate of the lead time for that particular cell type.
Study results
Henschke et al. (1) reported on the percent of all detected nodules that were found to be malignant within particular size categories. This was done separately for solid and for ground glass nodules (GGNs). As might be expected, the proportion of malignant nodules increased for each successive size category of baseline-detected solid nodules (Figure 1A). For nodules newly detected during annual repeat rounds, the proportion that were malignant was higher than seen in the baseline round in the lower size ranges, but was diminished in the higher ranges. It is logical that a new nodule may be more likely to represent a new lung cancer; however, if the new finding is particularly large it may be more likely to represent an inflammatory (benign) process.
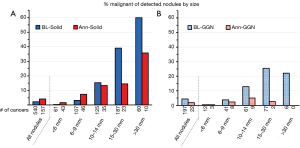
For GGNs (either pure ground glass or part-solid) found during a baseline round the percent that were malignant also increased with size, but was lower than for solid nodules (Figure 1B). Few newly detected GGNs during annual repeat rounds were found to be cancers, regardless of size. However, the number of GGNs was much lower than that of solid nodules, particularly in the higher size categories. Of note, the size was measured as the total size of the ground glass component (and as an average of the length and width). No information is provided on the size of any solid component of the GGNs.
Henschke also found that the proportion of indolent cancers was greater in the baseline round than the annual repeat rounds (Figure 2). This is reflected in a higher proportion of carcinoid tumors and adenocarcinomas presenting as GGNs, as well as in a decreased proportion of small cell lung cancer (SCLC). The indolent nature of carcinoid and adenocarcinomas presenting as GGN is also reflected in the estimate of the lead time due to the screening (how much sooner screening led to the detection before the development of symptoms). However, we should recognize that this lead time estimate is a calculated rough approximation; it should not be taken literally and confused with an actually demonstrated lead time.
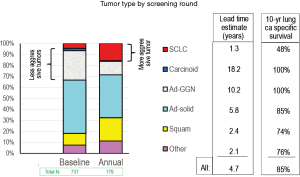
Henschke et al. (1) interpreted the results relative to the question of when is the probability of lung cancer high enough to trigger concern (further investigation or intervention) given its size and whether it is a baseline or annual screening round. They found that the results justified the I-ELCAP policy of concern for a baseline nodule of 6 mm or more, and 3 mm or more for a new nodule found by an annual repeat scan. This is based on the arguments that the chance of lung cancer is lower in baseline than in repeat scans for a given size, that a delay in investigating a small (e.g., 4 mm) nodule at baseline is less concerning because the proportion of indolent tumors is higher, a patient accrues a risk only once of a delay in work-up for a baseline finding as opposed to delays in work-ups of annual findings, and increasing the threshold size at baseline is particularly useful, given the far greater number of baseline nodules and thus the associated cost reduction.
Taking it a step further
Fundamentally, the interpretation of Henschke et al. (1) asks a one-size-fits-all question “Is it cancer?” I think the study suggests that we have to be more nuanced. Given the significant proportion of very indolent cancers, we must also ask “what sort of cancer is this” and “how will this cancer behave?” Very indolent tumors may take many years to impact a patient’s life; in such cases we must consider the balance between the progression of, for example, a ground glass adenocarcinoma and a patient’s comorbidities and competing causes of death. The study by Henschke et al. underscores this, by demonstrating the proportion of indolent tumors and the estimate of long lead times.
It is important to recognize indolent tumors and not to overreact. With respect to adenocarcinomas presenting as GGNs, prospective studies have shown that the majority do not progress significantly over 10 years (2-4), and other studies suggest that there may be genetically different types with some destined to progress and others not to (5,6). If we recognize very indolent cancers and manage them appropriately (balancing their rate and risk of progression with an individual’s general health and life expectancy), the issue of “overdiagnosis” due to screening disappears.
It is unfortunate that the study by Henschke et al. (1) reports on the total size of GGNs and provides no information about the size of the solid component. Many studies have consistently shown that the ground glass (or histologically the lepidic) component does not affect prognosis—it is the size of a solid (or histologically the invasive) component that matters (7-12). The current 8th edition of TNM stage classification assigns the T stage purely on the basis of the solid (clinical stage) or invasive (pathologic stage) component (13).
The substantial proportion of less aggressive tumors at baseline screening has other important practical implications. The salient point is not that it is the first scan, but that it is a scan without a recent previous scan. The nature of nodules noted by Henschke et al. probably fairly closely reflect the spectrum of aggressiveness seen when an individual undergoes an incidental chest CT scan (not in the context of a screening program). Furthermore, some have suggested that the interval between lung cancer screening rounds should be several years if the baseline scan shows nothing of concern. The longer the interval, the more a repeat round will have the altered spectrum of aggressiveness seen during the baseline round. In this regard, it is perhaps less important to focus on the proportion of indolent tumors (which we will see more frequently but ideally manage judiciously), but the decreased proportion of aggressive tumors that are found in an early stage. After all, the key to reducing lung cancer mortality through screening is to reduce the number of patients with more aggressive cancer that are diagnosed at an advanced stage. From this perspective, increasing the interval between screening rounds diminishes our ability to find the aggressive cancers early.
Summary
Screening for lung cancer is a complex interplay of many interrelated factors. Cancers detected in the setting of screening are not exactly the same as those detected in routine care; whether it is a baseline or a repeat screening round scan alters the spectrum of aggressiveness among detected tumors, as demonstrated in a recent study by Henschke et al. from the extensive I-ELCAP experience. Understanding how altering parameters of the screening process (e.g., interval between rounds, nodule size threshold, an individual’s risk of development of lung cancer, their competing comorbidities) affects multiple outcomes (e.g., what type of cancers are detected, stage shift, potential overdiagnosis) is important. With a well-informed, thoughtful and nuanced approach we can realize major benefits for patients at risk of developing lung cancer through low dose CT screening.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Henschke CI, Salvatore M, Cham M, et al. Baseline and annual report rounds of screening: implications for optimal regimens of screening. Eur Radiol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Kobayashi Y, Fukui T, Ito S, et al. How Long should small lung lesions of ground-glass opacity be followed? J Thorac Oncol 2013;8:309-14. [Crossref] [PubMed]
- Sawada S, Yamashita N, Sugimoto R, et al. Long-term outcomes of patients with ground-glass opacities detected using computed tomography. Chest 2017;151:308-15. [Crossref] [PubMed]
- Kakinuma R, Noguchi M, Ashizawa K, et al. Natural history of pulmonary subsolid nodules: a prospective multicenter study. J Thorac Oncol 2016;11:1012-28. [Crossref] [PubMed]
- Kobayashi Y, Mitsudomi T, Sakao Y, et al. Genetic features of pulmonary adenocarcinoma presenting with ground-glass nodules: the differences between nodules with and without growth. Ann Oncol 2015;26:156-61. [Crossref] [PubMed]
- Yatabe Y, Borczuk AC, Powell CA. Do all lung adenocarcinomas follow a stepwise progression? Lung Cancer 2011;74:7-11. [Crossref] [PubMed]
- Maeyashiki T, Suzuki K, Hattori A, et al. The size of consolidation on thin-section computed tomography is a better predictor of survival than the maximum tumour dimension in resectable lung cancer. Eur J Cardiothorac Surg 2013;43:915-8. [Crossref] [PubMed]
- Murakawa T, Konoeda C, Ito T, et al. The ground glass opacity component can be eliminated from the T-factor assessment of lung adenocarcinoma. Eur J Cardiothorac Surg 2013;43:925-32. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Prognostic significance of using solid versus whole tumor size on high-resolution computed tomography for predicting pathologic malignant grade of tumors in clinical stage IA lung adenocarcinoma: A multicenter study. J Thorac Cardiovasc Surg 2012;143:607-12. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Mimae T, et al. The prognostic role of pathologic invasive component size, excluding lepidic growth, in stage I lung adenocarcinoma. J Thorac Cardiovasc Surg 2013;146:580-5. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage i lung adenocarcinoma. J Thorac Oncol 2013;8:612-8. [Crossref] [PubMed]
- Sawabata N, Kanzaki R, Sakamoto T, et al. Clinical predictor of pre- or minimally invasive pulmonary adenocarcinoma: possibility of sub-classification of clinical T1a. Eur J Cardiothorac Surg 2014;45:256-61. [Crossref] [PubMed]
- Travis D, Asamura H, Bankier AA, et al. The IASLC lung cancer staging project: proposals for coding t categories for subsolid nodules and assessment of tumor size in part solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016;11:1204-23.


